Contribute To The Mass Of An Atom
Juapaving
Mar 17, 2025 · 6 min read
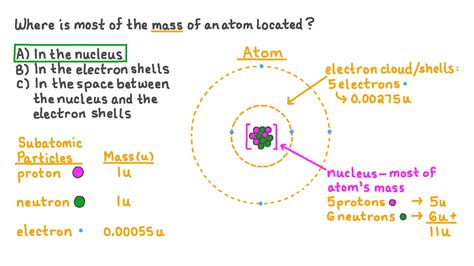
Table of Contents
Contributing to the Mass of an Atom: A Deep Dive into Protons, Neutrons, and Electrons
Understanding the mass of an atom is fundamental to comprehending chemistry and physics. While seemingly simple, the contribution of different subatomic particles to this mass reveals a fascinating interplay of forces and interactions. This article delves into the intricacies of atomic mass, exploring the dominant contributors and the nuances that influence its precise measurement.
The Major Players: Protons and Neutrons
The vast majority of an atom's mass is concentrated in its nucleus, residing within the protons and neutrons. These particles are collectively known as nucleons.
Protons: The Positively Charged Contributors
Protons, each carrying a single positive charge, contribute significantly to an atom's mass and its overall positive nuclear charge. A proton's mass is approximately 1.6726 x 10<sup>-27</sup> kg, a value often rounded to 1 atomic mass unit (amu). The number of protons in an atom's nucleus defines its atomic number and determines the element to which it belongs. For instance, all hydrogen atoms have one proton, all helium atoms have two, and so on. This is crucial for understanding the periodic table and the chemical properties of elements.
Neutrons: The Neutral Mass Boosters
Neutrons, as their name suggests, carry no electrical charge. Their mass is slightly larger than that of a proton, approximately 1.6749 x 10<sup>-27</sup> kg, also roughly 1 amu. Neutrons play a vital role in stabilizing the nucleus. In most atoms, the number of neutrons is roughly equal to the number of protons, particularly in lighter elements. However, the neutron-to-proton ratio varies across heavier elements to maintain nuclear stability. Isotopes, different forms of the same element with varying neutron numbers, illustrate this variation. For example, carbon-12 has six protons and six neutrons, while carbon-14 has six protons and eight neutrons. This difference in neutron number significantly impacts the atom's stability and potential for radioactive decay.
The Negligible Contribution: Electrons
Electrons, orbiting the nucleus, are significantly less massive than protons and neutrons. Their mass is approximately 9.1094 x 10<sup>-31</sup> kg, nearly 2000 times smaller than that of a proton. Consequently, electrons contribute negligibly to an atom's overall mass. While their mass is insignificant compared to protons and neutrons, electrons are crucial for chemical bonding and the atom's overall charge neutrality. The number of electrons in an electrically neutral atom equals the number of protons.
Mass Defect and Binding Energy: A Subtle Shift in Mass
The mass of an atom isn't simply the sum of the masses of its constituent protons, neutrons, and electrons. A phenomenon known as the mass defect comes into play. When nucleons combine to form a nucleus, a small amount of mass is converted into energy, according to Einstein's famous equation, E=mc². This energy, known as binding energy, holds the nucleus together, overcoming the repulsive forces between positively charged protons. The larger the binding energy per nucleon, the more stable the nucleus.
The mass defect is a subtle but crucial aspect of atomic mass. It explains why the measured mass of an atom is slightly less than the sum of the masses of its individual components. This seemingly small difference represents a substantial amount of energy released during nuclear reactions, as seen in nuclear fission and fusion.
Understanding Isotopes and Their Mass Numbers
Isotopes, variations of an element with the same number of protons but different numbers of neutrons, have different masses. The mass number of an atom represents the total number of protons and neutrons in its nucleus. For example, carbon-12 has a mass number of 12 (6 protons + 6 neutrons), while carbon-14 has a mass number of 14 (6 protons + 8 neutrons). The mass number provides a convenient way to distinguish between isotopes and is often used in nuclear chemistry and physics.
Measuring Atomic Mass: Beyond Simple Addition
Determining the precise mass of an atom is a complex undertaking, requiring sophisticated techniques like mass spectrometry. This method separates ions based on their mass-to-charge ratio, allowing for accurate mass measurements. The results are often expressed in atomic mass units (amu), where 1 amu is defined as one-twelfth the mass of a carbon-12 atom.
The mass of an atom, as reported in periodic tables, is often a weighted average of the masses of its naturally occurring isotopes. This average reflects the relative abundance of each isotope in a sample. For example, chlorine has two main isotopes, chlorine-35 and chlorine-37. The weighted average mass of chlorine reflects the proportions of these two isotopes found in nature.
The Role of Atomic Mass in Chemistry and Physics
Atomic mass plays a vital role in various scientific disciplines:
-
Stoichiometry: Accurate calculations in stoichiometry, the study of quantitative relationships in chemical reactions, rely on precise knowledge of atomic masses. This allows for determining reactant and product quantities in chemical processes.
-
Nuclear Physics: Understanding atomic mass is crucial in nuclear physics, particularly in studying nuclear reactions, decay processes, and the stability of atomic nuclei.
-
Nuclear Medicine: Isotopes with specific masses are essential in nuclear medicine for diagnostic imaging and therapeutic applications.
-
Cosmology: The abundance of isotopes in the universe provides valuable insights into stellar nucleosynthesis and the formation of elements in stars.
Beyond the Basics: Exploring Isobaric and Isotonic Nuclides
While isotopes share the same number of protons but differ in neutrons, other relationships exist among nuclides (atomic nuclei).
-
Isobars: Nuclides with the same mass number but different atomic numbers (and therefore different numbers of protons and neutrons) are called isobars. Isobars have similar masses but different chemical properties due to their differing numbers of protons.
-
Isotones: Nuclides with the same number of neutrons but different numbers of protons and therefore different atomic numbers are known as isotones. Isotones exhibit similar nuclear properties due to their common neutron count but distinct chemical behaviors due to their differing proton counts.
Conclusion: The Intricate World of Atomic Mass
The contribution to the mass of an atom is not a simple summation of its constituent particles. It involves a delicate balance of nuclear forces, mass defect, binding energy, and the relative abundance of isotopes. Understanding the nuances of atomic mass is pivotal for advancing our knowledge in chemistry, physics, and related fields. From stoichiometric calculations to understanding nuclear reactions and the evolution of the cosmos, the concept of atomic mass remains a fundamental pillar of modern science. Continued research and refinement of measurement techniques continue to unveil deeper insights into the complexities of atomic structure and behavior.
Latest Posts
Latest Posts
-
Does Carbon Follow The Octet Rule
Mar 18, 2025
-
Real Life Example Of A Scalene Triangle
Mar 18, 2025
-
How Do You Convert A Ratio Into A Percent
Mar 18, 2025
-
What Fahrenheit Is 32 Degrees Celsius
Mar 18, 2025
-
How Many Sides Is A Heptagon
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Contribute To The Mass Of An Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
