Chemical Bonds From Weakest To Strongest
Juapaving
Mar 23, 2025 · 7 min read
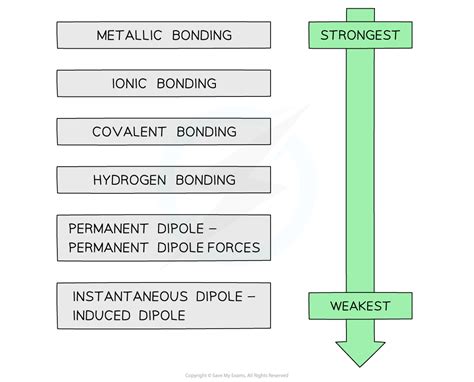
Table of Contents
Chemical Bonds: From Weakest to Strongest
Understanding chemical bonds is fundamental to grasping the properties and behavior of matter. From the simplest molecules to complex biological systems, the strength and type of chemical bonds dictate everything from melting points and boiling points to reactivity and solubility. This comprehensive guide will explore the diverse world of chemical bonds, arranging them from weakest to strongest, explaining their characteristics and providing examples of each.
The Spectrum of Chemical Bonds: A Hierarchy of Strength
Chemical bonds arise from the electrostatic attraction between atoms. This attraction can vary significantly in strength, resulting in a spectrum of bond types. We'll journey through this spectrum, starting with the weakest and progressing to the strongest bonds found in nature.
1. Van der Waals Forces: The Ephemeral Bonds
Van der Waals forces are the weakest type of intermolecular forces. They are temporary, fluctuating attractions that arise from temporary shifts in electron distribution within molecules. These shifts create instantaneous dipoles, inducing dipoles in neighboring molecules, resulting in a weak attraction. There are three main types of Van der Waals forces:
-
London Dispersion Forces (LDFs): These are the weakest of the Van der Waals forces and are present in all molecules, regardless of their polarity. They arise from temporary, instantaneous dipoles that occur due to the random movement of electrons. The larger the molecule (and therefore the more electrons), the stronger the LDFs. Examples include the attraction between nonpolar molecules like methane (CH₄) and noble gases.
-
Dipole-Dipole Forces: These forces occur between polar molecules possessing permanent dipoles. The positive end of one molecule is attracted to the negative end of another. Dipole-dipole interactions are stronger than LDFs but still relatively weak. Examples include interactions between molecules like hydrogen chloride (HCl) and acetone.
-
Hydrogen Bonding: While technically a special type of dipole-dipole interaction, hydrogen bonds deserve special mention due to their relatively strong nature among Van der Waals forces. They occur when a hydrogen atom bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) is attracted to another electronegative atom in a nearby molecule. Hydrogen bonding is crucial in many biological systems, contributing to the structure of proteins and DNA. Water's unique properties are largely due to extensive hydrogen bonding.
Strength Comparison (Van der Waals): London Dispersion Forces < Dipole-Dipole Forces < Hydrogen Bonds
2. Ionic Bonds: Electrostatic Attraction at its Core
Ionic bonds are formed through the electrostatic attraction between oppositely charged ions. This occurs when one atom loses one or more electrons (becoming a positively charged cation) and another atom gains those electrons (becoming a negatively charged anion). The resulting electrostatic attraction between the cation and anion forms the ionic bond.
Ionic bonds are generally stronger than Van der Waals forces but weaker than covalent bonds. Their strength is influenced by factors like the charge of the ions and the distance between them. Smaller ions with higher charges generally form stronger ionic bonds. Examples of ionic compounds include sodium chloride (NaCl), magnesium oxide (MgO), and potassium iodide (KI). These compounds typically have high melting and boiling points due to the strong electrostatic attractions.
3. Covalent Bonds: Sharing is Caring
Covalent bonds represent a different type of bonding mechanism compared to ionic bonds. Instead of electron transfer, atoms share electrons to achieve a stable electron configuration. This sharing results in a mutual attraction between the atoms, forming the covalent bond.
Covalent bonds are generally stronger than ionic bonds, particularly when dealing with multiple bonds (double or triple bonds). The strength of a covalent bond is influenced by factors such as the electronegativity difference between the atoms and the bond order (single, double, or triple bond). Electronegativity describes the ability of an atom to attract electrons in a bond.
Types of Covalent Bonds:
-
Nonpolar Covalent Bonds: These occur when two atoms of the same element or atoms with very similar electronegativities share electrons equally. Examples include the bonds in diatomic molecules like oxygen (O₂) and nitrogen (N₂).
-
Polar Covalent Bonds: These form when atoms with different electronegativities share electrons unequally. This results in a partial positive charge (δ+) on the less electronegative atom and a partial negative charge (δ-) on the more electronegative atom. Examples include the bonds in water (H₂O) and carbon dioxide (CO₂).
Strength Comparison (Covalent Bonds): Single Bonds < Double Bonds < Triple Bonds
4. Metallic Bonds: A Sea of Electrons
Metallic bonds are unique to metals and arise from the delocalization of valence electrons. In metallic structures, valence electrons are not associated with specific atoms but rather move freely throughout the metal lattice, forming a "sea" of electrons. This "sea" of electrons acts as a glue, holding the positively charged metal ions together.
The strength of metallic bonds varies depending on the metal's electron configuration and the number of valence electrons. Metals with more valence electrons generally form stronger metallic bonds. Metallic bonds are responsible for many characteristic properties of metals, such as their high electrical and thermal conductivity, malleability, and ductility. Examples include the bonds in copper (Cu), iron (Fe), and gold (Au).
5. Coordinate Covalent Bonds (Dative Bonds): A Special Case
Coordinate covalent bonds, also known as dative bonds, are a type of covalent bond where both electrons shared in the bond originate from the same atom. This is in contrast to typical covalent bonds where each atom contributes one electron. The atom that donates both electrons is called the donor atom, and the atom that accepts the electrons is called the acceptor atom. Coordinate covalent bonds are important in the formation of complex ions and some molecules. Ammonia-borane (NH₃BH₃) and metal complexes are prime examples.
Comparing Bond Strengths: A Summary Table
| Bond Type | Strength | Description | Example |
|---|---|---|---|
| Van der Waals Forces | Weakest | Temporary attractions due to electron fluctuations | Methane (CH₄) |
| Ionic Bonds | Moderate | Electrostatic attraction between oppositely charged ions | Sodium Chloride (NaCl) |
| Covalent Bonds | Strong | Sharing of electrons between atoms | Water (H₂O) |
| Metallic Bonds | Strong to Very Strong | Delocalized electrons in a sea of electrons holding metal ions together | Copper (Cu) |
| Coordinate Covalent | Similar to Covalent | One atom donates both electrons in the shared pair | Ammonia-borane (NH₃BH₃) |
Note: The strength of bonds is not absolute and can be influenced by several factors such as the size of atoms, their electronegativity, and the environment. The table above provides a general comparison.
Applications and Implications
Understanding the relative strengths of different chemical bonds has far-reaching implications across various scientific disciplines:
-
Material Science: The properties of materials, like their strength, conductivity, and melting point, are directly related to the types and strengths of chemical bonds present. This knowledge is crucial for designing new materials with specific properties.
-
Chemistry: Predicting the reactivity of molecules and their behavior in chemical reactions depends heavily on understanding the nature and strength of their bonds.
-
Biology: The structure and function of biological molecules, such as proteins and DNA, are largely determined by the types and strengths of chemical bonds, including hydrogen bonds and covalent bonds.
Conclusion
The world of chemical bonds is intricate and fascinating. From the ephemeral Van der Waals forces to the robust covalent and metallic bonds, the diverse range of bonding interactions drives the incredible variety and complexity we observe in the physical world. Understanding the relative strengths and characteristics of these bonds is crucial for comprehending the properties and behavior of matter across all scales, from individual molecules to macroscopic materials and complex biological systems. Further research and exploration continue to unveil deeper insights into the nuances of chemical bonding, leading to advancements across numerous fields.
Latest Posts
Latest Posts
-
All Of The Following Are True Except
Mar 25, 2025
-
How Many Kings Are In A Deck Of 52 Cards
Mar 25, 2025
-
Why Are Ionic Compounds Soluble In Water
Mar 25, 2025
-
Surface Area Of A Hemisphere Calculator
Mar 25, 2025
-
Put Numbers In Order From Least To Greatest
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Chemical Bonds From Weakest To Strongest . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
