Boiling Point Of Water Kelvin Scale
Juapaving
Mar 21, 2025 · 5 min read
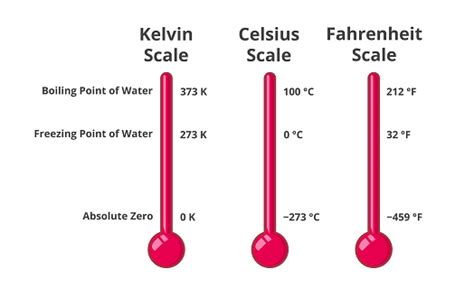
Table of Contents
Boiling Point of Water on the Kelvin Scale: A Deep Dive
The boiling point of water is a fundamental concept in science and everyday life. While we often cite it as 100° Celsius or 212° Fahrenheit, its representation on the Kelvin scale holds significant scientific importance. This article will delve deep into the boiling point of water on the Kelvin scale, exploring its definition, significance, factors influencing it, and its applications across various scientific disciplines.
Understanding the Kelvin Scale
Before diving into the boiling point of water specifically, let's establish a firm understanding of the Kelvin scale itself. Unlike the Celsius and Fahrenheit scales, which are relative scales with arbitrary zero points, the Kelvin scale is an absolute temperature scale. Its zero point, 0 Kelvin (0 K), represents absolute zero – the theoretical temperature at which all molecular motion ceases. This makes the Kelvin scale particularly useful in scientific calculations and thermodynamic processes.
Key Differences from Celsius and Fahrenheit
-
Absolute Zero: The Kelvin scale's defining feature is its absolute zero point, providing a true measure of thermal energy. Celsius and Fahrenheit have arbitrary zero points based on the freezing point of water (or a mixture of ice and salt in Fahrenheit's case).
-
Scale Intervals: The size of a degree on the Kelvin scale is identical to the size of a degree on the Celsius scale. This means a change of 1 Kelvin (1 K) is equivalent to a change of 1 degree Celsius (1 °C).
-
No Negative Temperatures: Since the Kelvin scale starts at absolute zero, there are no negative temperatures. This simplifies many scientific calculations and prevents potential ambiguities.
The Boiling Point of Water in Kelvin
The boiling point of water at standard atmospheric pressure (1 atmosphere or 101.325 kPa) is 373.15 Kelvin (373.15 K). This value is derived directly from the Celsius scale, where the boiling point is 100°C. The conversion between Celsius and Kelvin is straightforward:
K = °C + 273.15
Therefore, 100°C + 273.15 = 373.15 K.
Factors Affecting the Boiling Point of Water
While 373.15 K is the standard boiling point, several factors can influence the actual temperature at which water boils:
1. Atmospheric Pressure
This is perhaps the most significant factor. Higher atmospheric pressure increases the boiling point, while lower atmospheric pressure decreases it. At higher altitudes, where atmospheric pressure is lower, water boils at a lower temperature. This is why cooking times can be longer at high altitudes – the water isn't as hot. Conversely, pressure cookers operate at higher pressures, increasing the boiling point and allowing for faster cooking.
2. Impurities
The presence of dissolved impurities in water can subtly affect its boiling point. Generally, dissolved substances raise the boiling point, a phenomenon known as boiling point elevation. This effect is often minimal for common impurities in drinking water but becomes more significant in solutions with higher concentrations of dissolved solids.
3. Isotopic Composition
Water molecules are composed of hydrogen and oxygen atoms. However, hydrogen has isotopes (deuterium and tritium). Water enriched in heavier isotopes like deuterium (heavy water) will have a slightly higher boiling point than ordinary water.
The Significance of the Boiling Point in Kelvin
The boiling point of water in Kelvin holds immense significance across various scientific domains:
1. Thermodynamics
In thermodynamics, the Kelvin scale is crucial for defining and understanding concepts like enthalpy and entropy changes during phase transitions (like boiling). The precise boiling point at 373.15 K is a critical reference point for many thermodynamic calculations and models.
2. Chemistry
In chemistry, the boiling point is an important physical property used for substance identification and purification. The accurate Kelvin value provides consistent and comparable data across different experiments and research settings.
3. Physics
The boiling point plays a vital role in understanding heat transfer, phase changes, and the behavior of fluids. Its precise Kelvin measurement facilitates more accurate simulations and predictions in various physical systems.
4. Engineering
Engineers utilize the boiling point of water (and its dependence on pressure) in designing various systems, including steam engines, power plants, and cooling systems. Accurate knowledge of the boiling point across different pressure conditions is essential for optimizing performance and safety.
5. Meteorology
In meteorology, the boiling point is relevant to understanding atmospheric processes and weather phenomena. Variations in boiling point due to altitude and pressure changes directly affect weather patterns and cloud formation.
Applications of the Boiling Point Knowledge
Understanding the boiling point of water, especially expressed in Kelvin, has numerous practical applications:
-
Cooking: As mentioned earlier, altitude significantly affects the boiling point, impacting cooking times. Knowing this allows for adjustments in recipes and cooking techniques at higher elevations.
-
Steam Power Generation: Power plants rely on the efficient conversion of water to steam to generate electricity. Precise control of temperature (expressed in Kelvin) is crucial for maximizing efficiency and safety.
-
Sterilization: Boiling water is a common method of sterilization. Understanding the boiling point helps determine the appropriate temperature and time for effective sterilization.
-
Industrial Processes: Many industrial processes utilize steam or involve boiling liquids. Precise temperature control, often using Kelvin measurements, is critical for quality control and safety.
Advanced Concepts and Further Exploration
For those seeking a deeper understanding, several advanced concepts relate to the boiling point of water in Kelvin:
-
Critical Point: The critical point of water represents the temperature and pressure above which the distinction between liquid and gaseous phases disappears.
-
Clausius-Clapeyron Equation: This equation describes the relationship between the vapor pressure of a liquid and its temperature, enabling the calculation of boiling points at different pressures.
-
Phase Diagrams: Phase diagrams visually represent the different phases of a substance (solid, liquid, gas) as a function of temperature and pressure, offering a comprehensive view of the boiling point's relationship to other thermodynamic properties.
Conclusion
The boiling point of water at 373.15 K is far more than a simple numerical value; it represents a fundamental physical constant with widespread implications across various scientific disciplines and practical applications. Its significance extends beyond everyday observations, providing a cornerstone for understanding thermodynamics, chemistry, physics, and engineering principles. By comprehending the factors that influence the boiling point and its representation on the absolute Kelvin scale, we gain valuable insights into the behavior of water and its critical role in numerous natural and engineered systems. From understanding how altitude affects cooking to optimizing industrial processes, the precise knowledge of water's boiling point in Kelvin remains invaluable.
Latest Posts
Latest Posts
-
Words With Ie In Them 5 Letters
Mar 27, 2025
-
What Are The Pros Of Fossil Fuels
Mar 27, 2025
-
An Object Following A Straight Line Path At Constant Speed
Mar 27, 2025
-
Is Ice Cream Melting A Physical Change
Mar 27, 2025
-
Why Does Electronegativity Increase From Left To Right
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about Boiling Point Of Water Kelvin Scale . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
