Boiling Point Of Water In Kelvin Scale
Juapaving
Apr 06, 2025 · 5 min read
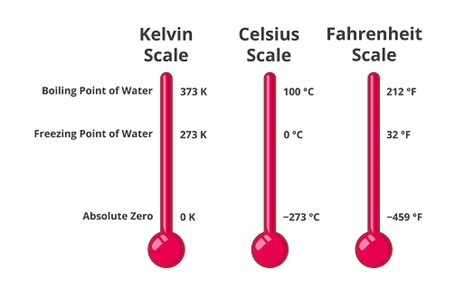
Table of Contents
Boiling Point of Water in Kelvin Scale: A Deep Dive
The boiling point of water, a seemingly simple concept, holds significant importance across various scientific disciplines and everyday life. Understanding this fundamental property, particularly when expressed in the Kelvin scale, unlocks a deeper understanding of thermodynamics, phase transitions, and the behavior of matter. This comprehensive article delves into the intricacies of water's boiling point in Kelvin, exploring its significance, influencing factors, and applications.
What is the Boiling Point of Water in Kelvin?
The boiling point of water at standard atmospheric pressure (1 atmosphere or 101.325 kPa) is 373.15 Kelvin (K). This is equivalent to 100 degrees Celsius (°C) or 212 degrees Fahrenheit (°F). It's crucial to note that the boiling point isn't a fixed constant; it's highly sensitive to changes in pressure. This is why high-altitude cooking requires adjustments to cooking times – the lower atmospheric pressure at higher altitudes results in a lower boiling point for water.
Understanding the Kelvin Scale
The Kelvin scale is an absolute thermodynamic temperature scale. Unlike Celsius and Fahrenheit, which are relative scales with arbitrary zero points, the Kelvin scale's zero point (0 K) represents absolute zero, the theoretical point at which all molecular motion ceases. This makes it particularly useful in scientific calculations involving temperature-dependent properties. The Kelvin scale is directly proportional to the Celsius scale; a change of 1 K is equivalent to a change of 1 °C. To convert Celsius to Kelvin, simply add 273.15.
Factors Affecting the Boiling Point of Water
Several factors influence the boiling point of water, deviating it from the standard 373.15 K. Understanding these factors is crucial for accurate scientific measurements and various industrial applications.
Pressure: The Dominant Factor
Pressure is the most significant factor influencing water's boiling point. As atmospheric pressure decreases, the boiling point decreases. This is because the reduced pressure allows water molecules to escape the liquid phase more easily. Conversely, an increase in pressure raises the boiling point, requiring more energy to overcome the stronger intermolecular forces. This principle is utilized in pressure cookers, where increased pressure leads to higher boiling temperatures, resulting in faster cooking times.
High-Altitude Cooking: A Practical Example
High-altitude cooking perfectly illustrates the pressure-boiling point relationship. At higher altitudes, the atmospheric pressure is lower, causing water to boil at a lower temperature. This means that food takes longer to cook because the temperature is lower. Recipes often need adjustments to account for this lower boiling point.
Impurities: Subtle but Significant
The presence of impurities in water, such as dissolved salts or other substances, can slightly elevate the boiling point. This phenomenon is known as boiling point elevation. The extent of the elevation depends on the concentration of the impurities. This effect is relatively small compared to the influence of pressure but is important in applications requiring high-purity water.
Isotopic Composition: A Minor Effect
Water molecules consist of hydrogen and oxygen atoms. However, hydrogen has isotopes: protium (¹H), deuterium (²H), and tritium (³H). The presence of heavier isotopes, like deuterium, can slightly increase the boiling point due to the increased mass of the water molecule. This effect is relatively small but measurable with precise instruments.
Significance of the Boiling Point in Kelvin
The boiling point of water in Kelvin holds immense significance in various fields:
Thermodynamics and Phase Transitions
Understanding the boiling point in Kelvin is fundamental to thermodynamics. It represents the temperature at which the Gibbs free energy of the liquid and gaseous phases of water are equal, marking the equilibrium between the two states. This concept is vital for understanding phase transitions and equilibrium processes.
Chemical Engineering and Industrial Processes
Many industrial processes rely on precise temperature control. Knowing the boiling point of water in Kelvin is critical for designing and optimizing processes involving steam generation, heat transfer, and chemical reactions involving water.
Meteorology and Climate Science
The boiling point of water is relevant to understanding atmospheric processes, including cloud formation, precipitation, and evaporation. Variations in atmospheric pressure and temperature directly impact the boiling point, affecting weather patterns and climate models.
Food Science and Culinary Arts
As discussed earlier, the boiling point of water plays a crucial role in cooking, especially at high altitudes. Understanding its dependence on pressure is essential for achieving consistent cooking results in different environments.
Applications Leveraging the Boiling Point in Kelvin
The knowledge of water's boiling point in Kelvin has practical applications in various domains:
Steam Generation for Power Plants
Power plants utilize the boiling point of water to generate steam, which drives turbines and produces electricity. Precise temperature control using the Kelvin scale ensures efficient and safe operation.
Sterilization and Disinfection
Boiling water is a common method of sterilization and disinfection. The high temperature at the boiling point effectively kills many microorganisms. Understanding the boiling point ensures effective sterilization.
Chemical Reactions and Processes
Many chemical reactions involve water as a solvent or reactant. Knowing the boiling point in Kelvin is crucial for controlling reaction rates and preventing unwanted side reactions.
Calibration of Thermometers and Sensors
The boiling point of water serves as a reference point for calibrating thermometers and other temperature-measuring devices. Its precise value in Kelvin provides a standard for accurate temperature measurement.
Conclusion: The Boiling Point – More Than Just a Number
The boiling point of water, particularly when expressed in the Kelvin scale, is far more than a simple numerical value. It's a fundamental property with wide-ranging implications across numerous scientific and practical applications. Understanding the factors that influence this boiling point and its relevance in different contexts is crucial for advancements in various fields, from energy production to food science and beyond. The seemingly simple act of water boiling hides a rich tapestry of thermodynamic principles and practical applications that continue to shape our understanding of the world around us. Further research into the nuances of this fundamental property promises to unlock even more insights and innovative applications in the future. This deep understanding of the boiling point of water in Kelvin underscores the importance of precise measurement and the interconnectedness of seemingly disparate scientific concepts.
Latest Posts
Latest Posts
-
5 Letter Words Start With Ad
Apr 07, 2025
-
The Sum Of Consecutive Odd Numbers Of 135
Apr 07, 2025
-
Moment Of Inertia Circular Cross Section
Apr 07, 2025
-
How Many Yards Are In 12 Feet
Apr 07, 2025
-
How Many Electrons Are In A Neutral Lithium Atom
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about Boiling Point Of Water In Kelvin Scale . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
