How Many Electrons Are In A Neutral Lithium Atom
Juapaving
Apr 07, 2025 · 6 min read
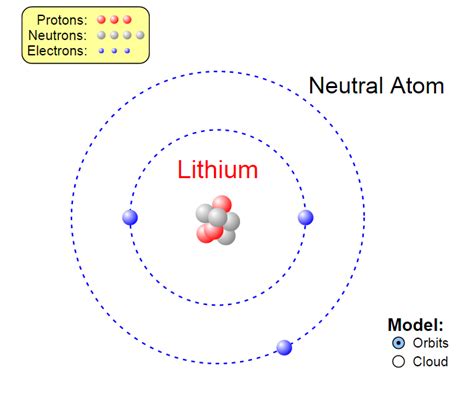
Table of Contents
How Many Electrons Are in a Neutral Lithium Atom? A Deep Dive into Atomic Structure
Understanding the number of electrons in a neutral lithium atom is fundamental to grasping basic chemistry and atomic physics. While the answer itself is simple, the journey to understanding why that number is correct reveals a wealth of information about atomic structure, electron shells, and the periodic table. This comprehensive guide delves into the intricacies of lithium's atomic makeup, explaining not only the electron count but also the underlying principles that govern it.
The Simple Answer: 3 Electrons
A neutral lithium atom contains three electrons. This seemingly straightforward answer is a cornerstone of its chemical properties and its placement on the periodic table. But let's explore the "why" behind this number.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Atoms are the fundamental building blocks of matter. They consist of three subatomic particles:
- Protons: Positively charged particles found in the atom's nucleus.
- Neutrons: Neutrally charged particles also located in the nucleus.
- Electrons: Negatively charged particles that orbit the nucleus in shells or energy levels.
The number of protons in an atom's nucleus defines its atomic number and determines the element. For lithium, the atomic number is 3, meaning it has three protons. In a neutral atom, the number of electrons is equal to the number of protons, maintaining a balance of positive and negative charge. This is crucial because atoms generally prefer to be electrically neutral.
Lithium's Position on the Periodic Table
Lithium (Li) resides in the second row, first column of the periodic table. Its placement provides valuable clues about its electronic configuration.
- Period (Row): The period number indicates the highest principal energy level (shell) occupied by an electron in its ground state. Lithium's position in the second row means its electrons occupy up to the second energy level (n=2).
- Group (Column): The group number (for the representative elements) generally corresponds to the number of valence electrons – electrons in the outermost shell. Lithium's position in Group 1 signifies it has one valence electron.
Electron Shells and Subshells: A More Detailed Look
Electrons don't simply orbit the nucleus randomly; they occupy specific energy levels called shells. These shells are further divided into subshells, which are characterized by their shape and the number of orbitals they contain.
- Shell (Principal Quantum Number, n): Describes the energy level and distance from the nucleus. The closer the shell is to the nucleus, the lower its energy.
- Subshell (Azimuthal Quantum Number, l): Describes the shape of the electron's orbital within a shell. The subshells are designated as s, p, d, and f.
- Orbital (Magnetic Quantum Number, ml): Specifies the orientation of the orbital in space. Each subshell contains a specific number of orbitals.
For lithium:
- First Shell (n=1): Contains only one subshell, the 1s subshell, which holds a maximum of two electrons.
- Second Shell (n=2): Contains two subshells: the 2s subshell (holds up to two electrons) and the 2p subshell (holds up to six electrons).
Therefore, the electronic configuration of lithium is 1s²2s¹. This means:
- Two electrons fill the 1s subshell.
- One electron occupies the 2s subshell.
This configuration explains why lithium has three electrons and why it's so reactive.
The Significance of the Valence Electron
The single electron in the 2s subshell is lithium's valence electron. Valence electrons are the outermost electrons and are primarily responsible for an element's chemical reactivity. Because lithium has only one valence electron, it readily loses this electron to achieve a stable, filled outer shell configuration, similar to that of the noble gas helium. This makes lithium highly reactive, readily forming ionic bonds with other elements.
Ions: What Happens When Lithium Loses an Electron?
When lithium loses its valence electron, it becomes a lithium ion (Li⁺). This ion has a positive charge because it now has one more proton than electron (3 protons and 2 electrons). The loss of the valence electron leads to a stable electron configuration, fulfilling the octet rule (having a full outer shell of electrons).
The formation of Li⁺ is a crucial aspect of lithium's chemistry. This ion is involved in numerous chemical reactions and is found in many compounds, including lithium batteries, which are prevalent in modern technology.
Isotopes: Variations in Neutron Count
While the number of protons and electrons in a neutral lithium atom are fixed (3 each), the number of neutrons can vary. These variations are called isotopes. Lithium has two naturally occurring stable isotopes:
- Lithium-6 (⁶Li): Contains 3 protons and 3 neutrons.
- Lithium-7 (⁷Li): Contains 3 protons and 4 neutrons.
Isotopes have the same number of protons and electrons, but different numbers of neutrons, leading to variations in their mass. The chemical properties of isotopes are largely similar, but their physical properties can differ.
Quantum Mechanics and Electron Behavior
The model presented above simplifies the complex reality of electron behavior. Quantum mechanics provides a more accurate, albeit more mathematically challenging, description of electron orbitals and energy levels. Quantum numbers (principal, azimuthal, magnetic, and spin) are used to describe the properties of each electron, showcasing the wave-particle duality of electrons. Instead of precise orbits, electrons exist in orbitals that represent probability distributions of their location.
Applications of Lithium and its Chemistry
The unique properties of lithium, stemming from its electronic configuration and reactivity, have led to its widespread application in various fields:
- Batteries: Lithium-ion batteries are ubiquitous in portable electronics, electric vehicles, and grid-scale energy storage. The high energy density and relatively low weight of lithium make it ideal for battery applications.
- Lubricants: Lithium-based greases are used as high-temperature lubricants due to their excellent thermal stability and resistance to oxidation.
- Ceramics and Glass: Lithium compounds are added to ceramics and glass to improve their strength, durability, and thermal resistance.
- Medicine: Lithium salts are used as mood stabilizers in the treatment of bipolar disorder.
Conclusion: A Deeper Understanding of Lithium
The simple answer – three electrons – opens the door to a rich understanding of atomic structure, chemical reactivity, and the periodic table. The electronic configuration of lithium, its valence electron, and the implications of its ionic form explain its unique properties and its widespread applications. By exploring these concepts, we gain a deeper appreciation for the fundamental principles governing the behavior of matter at the atomic level. The journey from a simple number to a complex understanding underscores the power of scientific inquiry and the interconnectedness of various scientific fields. Further exploration into quantum mechanics and nuclear physics will reveal even more intricate details about the nature of lithium and its atoms.
Latest Posts
Latest Posts
-
How Much Atp Does Electron Transport Chain Produce
Apr 09, 2025
-
Words That Have Az In It
Apr 09, 2025
-
8 Cups Is How Many Quarts
Apr 09, 2025
-
How Many Congruent Sides Does A Rhombus Have
Apr 09, 2025
-
What Is A Polynomial In Standard Form
Apr 09, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Are In A Neutral Lithium Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.