Basic Building Blocks Of All Matter.
Juapaving
Mar 21, 2025 · 6 min read
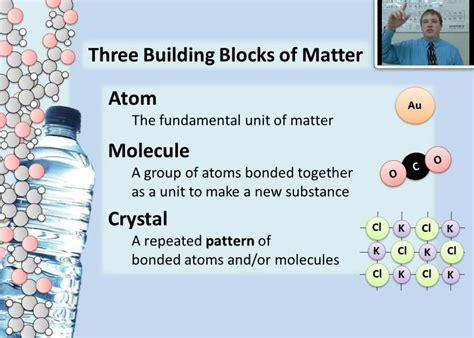
Table of Contents
The Basic Building Blocks of All Matter: A Deep Dive into Atoms, Elements, and Beyond
The universe, in all its breathtaking complexity, is fundamentally constructed from a surprisingly small number of basic building blocks. Understanding these fundamental components is crucial to grasping the nature of reality itself. This article delves into the fascinating world of atoms, elements, and the subatomic particles that make up everything we see, touch, and interact with. We'll explore their properties, interactions, and the broader implications of their existence.
Atoms: The Indivisible Units?
For centuries, philosophers and scientists pondered the fundamental nature of matter. The Greek philosopher Democritus proposed the concept of atomos, meaning "indivisible," suggesting that matter was composed of tiny, indestructible particles. While his idea was remarkably prescient, it lacked the experimental evidence needed for widespread acceptance. It wasn't until the late 19th and early 20th centuries that the atomic theory gained strong experimental support.
The Atomic Model's Evolution:
The understanding of the atom has evolved dramatically over time. Early models, like the "plum pudding" model proposed by J.J. Thomson, depicted a positively charged sphere with negatively charged electrons embedded within. However, this model was superseded by Ernest Rutherford's groundbreaking gold foil experiment, which revealed the atom's largely empty space with a dense, positively charged nucleus at its center. This led to the nuclear model of the atom.
Niels Bohr further refined this model by introducing the concept of electron shells or energy levels. Electrons orbit the nucleus in specific energy levels, and transitions between these levels involve the absorption or emission of energy in the form of light. This model successfully explained the discrete spectral lines observed in the emission spectra of elements.
The current understanding, based on quantum mechanics, is far more complex. The quantum mechanical model describes electrons not as orbiting particles but as existing in a cloud of probability around the nucleus. This probability cloud represents the likelihood of finding an electron in a particular location at any given time. This model accurately predicts the behavior of atoms and their interactions.
Elements: Defined by Atomic Number
Elements are pure substances consisting of only one type of atom. Each element is uniquely defined by its atomic number, which represents the number of protons in its nucleus. The number of protons determines the element's identity and its chemical properties. For instance, an atom with one proton is hydrogen, while an atom with six protons is carbon.
The Periodic Table: Organizing the Elements
The periodic table is a powerful tool for organizing and understanding the elements. It arranges elements in rows (periods) and columns (groups) based on their atomic number and electron configuration. Elements within the same group share similar chemical properties due to their similar outer electron arrangements. This organization highlights the periodic trends in properties like electronegativity, ionization energy, and atomic radius.
Understanding the periodic table is crucial for predicting the chemical behavior of elements and their compounds.
Subatomic Particles: Delving Deeper
Atoms themselves are composed of even smaller fundamental particles:
- Protons: Positively charged particles residing in the nucleus. The number of protons defines the element.
- Neutrons: Neutrally charged particles also found in the nucleus. They contribute to the atom's mass but not its charge. Isotopes of an element have the same number of protons but differ in the number of neutrons.
- Electrons: Negatively charged particles that orbit the nucleus. Their number determines the atom's overall charge and its chemical reactivity.
Quarks and Leptons: The Fundamental Building Blocks
Even protons and neutrons aren't fundamental. They are composed of even smaller particles called quarks. Quarks are fundamental particles that interact via the strong force, which holds the nucleus together. There are six types of quarks: up, down, charm, strange, top, and bottom. Protons and neutrons are each made up of three quarks.
Leptons are another class of fundamental particles, including electrons and neutrinos. Leptons are not affected by the strong force and are involved in weak interactions.
These fundamental particles, along with mediating particles like bosons (photons, gluons, W and Z bosons), constitute the Standard Model of particle physics, a framework that successfully describes the fundamental forces and particles of nature.
Isotopes and Radioactive Decay
Isotopes are atoms of the same element with the same number of protons but differing numbers of neutrons. This variation in neutron number affects the atom's mass but not its chemical properties. Some isotopes are radioactive, meaning their nuclei are unstable and decay over time, emitting radiation in the process. Radioactive decay is a crucial process in various fields, including nuclear medicine, radiocarbon dating, and nuclear energy.
Chemical Bonding: Atoms Joining Forces
Atoms interact with each other through chemical bonds, forming molecules and compounds. These bonds involve the sharing or transfer of electrons between atoms.
Types of Chemical Bonds:
- Ionic Bonds: Formed by the transfer of electrons from one atom to another, resulting in the formation of ions (charged atoms) held together by electrostatic attraction.
- Covalent Bonds: Formed by the sharing of electrons between atoms. Covalent bonds are strong and are responsible for the formation of most organic molecules.
- Metallic Bonds: Found in metals, where electrons are delocalized and shared among many atoms, resulting in a "sea" of electrons that accounts for the high electrical conductivity of metals.
The Implications of Understanding Matter's Building Blocks
Our understanding of the basic building blocks of matter has profound implications across numerous fields:
- Medicine: Radioactive isotopes are used in medical imaging and cancer therapy. Understanding chemical bonding is essential for developing new drugs and understanding biological processes.
- Materials Science: The properties of materials are determined by their atomic structure and bonding. This knowledge enables the design and synthesis of new materials with specific properties.
- Energy Production: Nuclear power plants harness the energy released from nuclear fission, a process involving the splitting of atomic nuclei.
- Electronics: The behavior of electrons in semiconductors is crucial for the functioning of electronic devices.
- Cosmology and Astrophysics: Understanding the fundamental particles and forces allows us to model the evolution of the universe and the formation of stars and galaxies.
Conclusion: An Ongoing Journey
The exploration of matter's basic building blocks is a journey that continues to this day. While the Standard Model provides a remarkably successful framework for understanding the universe at the fundamental level, many questions remain unanswered. Scientists continue to investigate the nature of dark matter and dark energy, search for new particles, and strive for a more complete and unified understanding of the fundamental forces governing the universe. The journey of understanding the basic building blocks of all matter is a testament to human curiosity and our relentless pursuit of knowledge. As our understanding deepens, so too will our ability to manipulate and utilize the fundamental forces of nature for the betterment of humankind. This ongoing investigation promises to unlock further wonders and technological advancements in the decades and centuries to come. The universe, with its intricate structure and fundamental components, remains a captivating subject, inviting further exploration and discovery.
Latest Posts
Latest Posts
-
Is 15 A Multiple Of 5
Mar 28, 2025
-
Where Is Most Of The Atp Made During Cellular Respiration
Mar 28, 2025
-
Calculate Water Pressure From Tank Height
Mar 28, 2025
-
What Numbers Can Go Into 27
Mar 28, 2025
-
Write 66 As A Product Of Prime Factors
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Basic Building Blocks Of All Matter. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
