Balanced Equation For Hydrochloric Acid And Sodium Hydroxide
Juapaving
Mar 10, 2025 · 6 min read
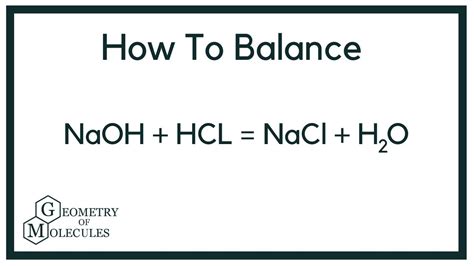
Table of Contents
The Balanced Equation for Hydrochloric Acid and Sodium Hydroxide: A Deep Dive
The reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) is a classic example of a neutralization reaction, a fundamental concept in chemistry. Understanding this reaction, its balanced equation, and its implications is crucial for students and professionals alike. This comprehensive guide will delve into the specifics of this reaction, exploring its balanced equation, the underlying principles, and its practical applications.
Understanding the Reactants: HCl and NaOH
Before diving into the reaction itself, let's briefly examine the properties of the two reactants: hydrochloric acid and sodium hydroxide.
Hydrochloric Acid (HCl)
Hydrochloric acid, also known as muriatic acid, is a strong, highly corrosive mineral acid. It's a solution of hydrogen chloride (HCl) in water. Its strong acidic nature stems from its complete dissociation in water, releasing hydrogen ions (H⁺) and chloride ions (Cl⁻). This high concentration of H⁺ ions is responsible for its acidic properties, like its ability to lower pH and react with bases. HCl is widely used in various industrial processes, including metal cleaning, leather processing, and food production.
Sodium Hydroxide (NaOH)
Sodium hydroxide, also known as caustic soda or lye, is a strong alkali (base). It's a white crystalline solid that readily dissolves in water, releasing sodium ions (Na⁺) and hydroxide ions (OH⁻). The high concentration of hydroxide ions (OH⁻) is responsible for its alkaline properties, including a high pH and the ability to neutralize acids. NaOH is extensively used in numerous applications, including soap making, paper production, and drain cleaning.
The Neutralization Reaction: HCl + NaOH
The reaction between HCl and NaOH is a classic acid-base neutralization reaction. In this reaction, the hydrogen ions (H⁺) from the acid react with the hydroxide ions (OH⁻) from the base to form water (H₂O). The remaining ions, sodium (Na⁺) and chloride (Cl⁻), combine to form sodium chloride (NaCl), commonly known as table salt.
The Balanced Chemical Equation
The balanced chemical equation for this reaction is:
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
This equation indicates that one mole of hydrochloric acid reacts with one mole of sodium hydroxide to produce one mole of sodium chloride and one mole of water. The "(aq)" denotes that the substance is dissolved in water (aqueous solution), while "(l)" indicates it's in the liquid state. The balanced equation ensures that the number of atoms of each element is equal on both sides of the equation, adhering to the law of conservation of mass.
Understanding the Balancing Process
Balancing a chemical equation is crucial because it reflects the stoichiometry of the reaction – the quantitative relationship between reactants and products. In this case, the equation is already balanced, meaning the number of atoms of each element is the same on both sides. For instance:
- Hydrogen (H): One hydrogen atom on each side.
- Chlorine (Cl): One chlorine atom on each side.
- Sodium (Na): One sodium atom on each side.
- Oxygen (O): One oxygen atom on each side.
This balance ensures that the reaction follows the fundamental principle of conservation of mass: matter is neither created nor destroyed in a chemical reaction.
The Ionic Equation and Net Ionic Equation
For a more detailed understanding of the reaction, we can also express it using ionic and net ionic equations.
The Ionic Equation
The ionic equation shows all the ions present in the solution before and after the reaction. It expands the balanced equation, breaking down the aqueous compounds into their constituent ions:
H⁺(aq) + Cl⁻(aq) + Na⁺(aq) + OH⁻(aq) → Na⁺(aq) + Cl⁻(aq) + H₂O(l)
The Net Ionic Equation
The net ionic equation simplifies the ionic equation by removing spectator ions. Spectator ions are ions that are present on both sides of the equation and do not participate in the reaction. In this case, Na⁺ and Cl⁻ are spectator ions. The net ionic equation represents the actual chemical change occurring:
H⁺(aq) + OH⁻(aq) → H₂O(l)
This equation highlights that the fundamental reaction is the combination of hydrogen ions and hydroxide ions to form water.
Practical Applications and Significance
The neutralization reaction between HCl and NaOH has numerous practical applications across various fields:
-
Acid-Base Titrations: This reaction is fundamental to acid-base titrations, a quantitative analytical technique used to determine the concentration of an unknown acid or base. By carefully measuring the volume of NaOH required to neutralize a known volume of HCl, the concentration of HCl can be precisely determined.
-
Chemical Synthesis: The reaction can be used in controlled chemical syntheses where precise pH control is crucial. Adding HCl or NaOH can adjust the pH of a reaction mixture, influencing the rate and direction of the reaction.
-
Wastewater Treatment: In wastewater treatment plants, this reaction helps neutralize acidic or alkaline wastewater before it's released into the environment. This neutralization prevents damage to aquatic ecosystems and ensures environmental safety.
-
Industrial Processes: Many industrial processes require careful pH control, and the neutralization reaction between HCl and NaOH is often employed to achieve this.
-
Food and Beverage Industry: The reaction plays a role in maintaining the pH of various food and beverage products, ensuring their quality and stability.
Safety Precautions
Both HCl and NaOH are corrosive substances and require careful handling. Always wear appropriate safety equipment, including gloves, goggles, and a lab coat, when working with these chemicals. In case of accidental contact, immediately rinse the affected area with plenty of water and seek medical attention if necessary.
Further Exploration: Factors Affecting the Reaction
Several factors can affect the rate and extent of the HCl and NaOH reaction:
-
Concentration of Reactants: Higher concentrations of HCl and NaOH will lead to a faster reaction rate due to increased collision frequency between the reacting ions.
-
Temperature: Increasing the temperature generally increases the reaction rate, as it provides more kinetic energy to the reacting ions, increasing the likelihood of successful collisions.
-
Presence of Catalysts: While not commonly used in this specific reaction, catalysts can potentially increase the reaction rate by lowering the activation energy.
Conclusion
The reaction between hydrochloric acid and sodium hydroxide is a quintessential example of an acid-base neutralization reaction. Understanding its balanced equation, the underlying principles, and its practical applications is fundamental to grasping key concepts in chemistry and related fields. From acid-base titrations to industrial processes and environmental remediation, this reaction plays a significant role in various aspects of our lives. Always remember to prioritize safety when working with these corrosive chemicals. By understanding the intricacies of this reaction, we can better appreciate the power and precision of chemistry.
Latest Posts
Latest Posts
-
How Are Frequency And Period Related To Each Other
Mar 10, 2025
-
Animal With The Fastest Reaction Time
Mar 10, 2025
-
Is 42 A Prime Or Composite Number
Mar 10, 2025
-
The Strength Of An Electromagnet Is Primarily Proportional To Its
Mar 10, 2025
-
Oxidation State Of Nitrogen In Ammonia
Mar 10, 2025
Related Post
Thank you for visiting our website which covers about Balanced Equation For Hydrochloric Acid And Sodium Hydroxide . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
