Are Ionic Compounds Solid At Room Temperature
Juapaving
Mar 26, 2025 · 6 min read
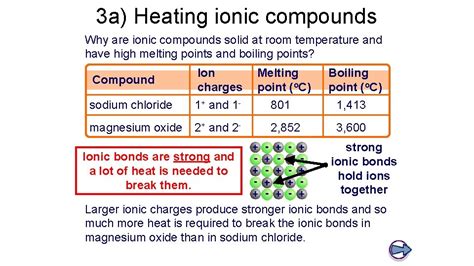
Table of Contents
Are Ionic Compounds Solid at Room Temperature? Exploring the Properties of Ionic Bonds
Ionic compounds, formed through the electrostatic attraction between oppositely charged ions, exhibit fascinating properties, one of the most prominent being their tendency to exist as solids at room temperature. This isn't simply a coincidence; it's a direct consequence of the strong forces holding the ions together within their crystal lattice structures. This article delves deep into the reasons behind this phenomenon, exploring the nature of ionic bonds, crystal lattices, and the factors influencing the melting and boiling points of ionic compounds. We'll also examine exceptions to this rule and discuss the implications of these properties in various applications.
Understanding Ionic Bonds: The Foundation of Solid Structures
At the heart of this discussion lies the ionic bond itself. This powerful bond arises from the electrostatic attraction between a positively charged ion (cation) and a negatively charged ion (anion). This attraction is significantly stronger than the weaker intermolecular forces found in covalent or metallic compounds. The formation of these ions typically involves a transfer of electrons, with highly electronegative atoms (like halogens) gaining electrons and becoming negatively charged anions, and less electronegative atoms (like alkali metals) losing electrons and becoming positively charged cations. This electron transfer leads to a stable octet electron configuration for both ions, fulfilling the octet rule and achieving lower overall energy states. The resulting electrostatic force between these oppositely charged species is substantial.
The Role of Coulomb's Law
The strength of the ionic bond can be quantitatively understood using Coulomb's Law: F = k * (q1 * q2) / r². This equation shows that the force (F) of attraction is directly proportional to the magnitudes of the charges (q1 and q2) of the ions and inversely proportional to the square of the distance (r) between them. This means that larger charges and shorter distances result in stronger ionic bonds. Therefore, compounds formed with ions carrying higher charges will have stronger bonds and higher melting points.
Crystal Lattices: The Ordered Arrangement of Ions
The incredibly strong electrostatic attractions between ions aren't random; they lead to a highly ordered, three-dimensional arrangement known as a crystal lattice. In this lattice structure, cations and anions are arranged in a repeating pattern that maximizes attractive forces and minimizes repulsive forces. The specific arrangement depends on the relative sizes and charges of the ions involved. Common lattice structures include cubic, tetragonal, orthorhombic, rhombohedral, monoclinic, and hexagonal.
Maximizing Attraction, Minimizing Repulsion
The arrangement in the crystal lattice is crucial for stability. The structure is optimized to ensure that each cation is surrounded by as many anions as possible, and vice-versa. This arrangement maximizes the attractive forces between oppositely charged ions while minimizing the repulsive forces between like-charged ions. The strong, multidirectional attractions within this highly organized structure contribute significantly to the solid nature of ionic compounds at room temperature. The energy required to overcome these numerous bonds is substantial, explaining the high melting and boiling points.
High Melting and Boiling Points: A Consequence of Strong Bonds
The high melting and boiling points of ionic compounds are a direct consequence of the strong electrostatic forces within the crystal lattice. To melt or boil an ionic compound, a significant amount of energy must be supplied to overcome these strong attractions and break the ionic bonds. This energy is much higher than that required to overcome the weaker intermolecular forces in covalent or molecular compounds, leading to significantly higher melting and boiling points for ionic substances. This is why most ionic compounds are solids at room temperature; the thermal energy at room temperature is insufficient to disrupt the strong lattice structure.
Factors Influencing Melting and Boiling Points
Several factors contribute to the variations in melting and boiling points among different ionic compounds:
-
Charge of the ions: Higher charges lead to stronger electrostatic attractions and therefore higher melting and boiling points. For example, MgO (Mg²⁺ and O²⁻) has a much higher melting point than NaCl (Na⁺ and Cl⁻).
-
Size of the ions: Smaller ions allow for closer proximity, resulting in stronger electrostatic attractions and higher melting and boiling points.
-
Lattice structure: The specific arrangement of ions in the crystal lattice can also influence the strength of the overall bonding and hence the melting and boiling point. More efficient packing arrangements generally lead to higher melting points.
Exceptions to the Rule: Factors Affecting Solid State
While the vast majority of ionic compounds are solid at room temperature, there are some exceptions. These exceptions highlight the interplay of several factors beyond the simple ionic bonding:
-
Polarity and hydrogen bonding: In some cases, the presence of polar bonds or hydrogen bonding can influence the properties. While the primary bonding is ionic, these secondary interactions can affect the overall intermolecular forces and the melting point.
-
Hydration: Certain ionic compounds readily dissolve in water, forming hydrated ions. These hydrated ions are surrounded by water molecules, which can weaken the overall ionic interactions, potentially leading to lower melting points or even liquid states at room temperature.
-
Ionic size and charge density: While smaller ions generally lead to higher melting points, excessively small or highly charged ions can lead to significant polarization and covalent character in the bonding, thereby reducing the strength of the purely ionic interactions.
-
Coordination number: The number of ions surrounding a given ion within the lattice structure (coordination number) affects the strength of the interactions. Certain arrangements can lead to weaker overall lattice energy, affecting the melting point.
Applications Leveraging the Properties of Ionic Compounds
The solid nature and high melting points of ionic compounds are exploited in numerous applications:
-
Ceramics: Many ceramics are based on ionic compounds and rely on their high melting points and strength for structural integrity at high temperatures.
-
Refractory materials: These materials withstand extremely high temperatures and often utilize ionic compounds with exceptionally strong lattice energies.
-
Salts and electrolytes: Ionic compounds are fundamental in numerous applications, including the use of salts for food preservation and in electrolytes for batteries and other electrochemical systems.
-
Construction materials: Certain ionic compounds contribute to the strength and durability of concrete and other construction materials.
-
Pharmaceuticals: Many pharmaceuticals are salts of organic molecules, where the ionic character plays a role in bioavailability and stability.
Conclusion: The Dominance of Ionic Bonding in Solid State
The solid nature of most ionic compounds at room temperature is a testament to the strength and stability of ionic bonds and their ordered crystal lattice arrangements. The powerful electrostatic forces between oppositely charged ions, governed by Coulomb's Law, require significant energy to overcome. This leads to the characteristically high melting and boiling points. While there are exceptions to this rule, largely due to the influence of secondary bonding or other factors, the dominant trend is clear: ionic compounds are fundamentally solid at room temperature due to the strength of the ionic interactions within their crystalline structure. Understanding this relationship is fundamental to appreciating their diverse applications and remarkable properties. The structure-property relationship in ionic compounds continues to be an area of ongoing research, continually expanding our understanding of these fascinating materials.
Latest Posts
Latest Posts
-
Heat Transfer In Liquids And Gases Takes Place By
Mar 29, 2025
-
What Is The Lcm For 3 And 4
Mar 29, 2025
-
What Percent Of 2 Is 3
Mar 29, 2025
-
Which Organelle Is Found Only In Plant Cells
Mar 29, 2025
-
What Is One Eighth As A Percentage
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Are Ionic Compounds Solid At Room Temperature . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
