Are Antiparalell Beta Sheets Mrore Stable
Juapaving
Mar 04, 2025 · 5 min read
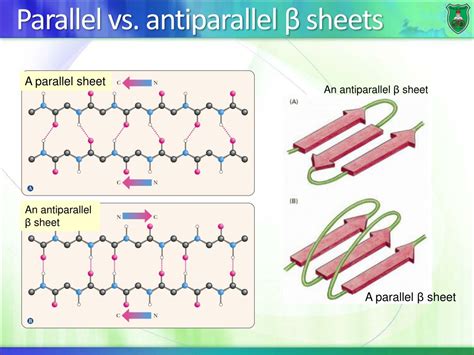
Table of Contents
Are Antiparallel Beta Sheets More Stable? Exploring the Energetics of Beta-Sheet Structure
Beta-sheets are fundamental secondary structures in proteins, contributing significantly to their overall stability and function. These structures are formed by hydrogen bonds between the backbone amide and carbonyl groups of adjacent polypeptide strands. While both parallel and antiparallel arrangements exist, the question of which is more stable has been a subject of ongoing investigation and debate. This article delves into the intricacies of beta-sheet stability, focusing on the comparative energetics of parallel and antiparallel arrangements. We'll explore the various factors contributing to this stability difference, examining experimental evidence and computational modeling to provide a comprehensive understanding.
The Fundamentals of Beta-Sheet Structure
Before diving into the stability comparison, it's crucial to understand the basic architecture of beta-sheets. Beta-sheets consist of extended polypeptide chains arranged side-by-side, forming a pleated sheet-like structure. These strands are linked by hydrogen bonds between the backbone amide (N-H) group of one strand and the carbonyl (C=O) group of an adjacent strand.
Parallel vs. Antiparallel Arrangements: A Structural Comparison
The key difference lies in the orientation of the participating strands.
-
Antiparallel Beta-Sheets: In this configuration, the adjacent strands run in opposite directions (N-terminus to C-terminus and vice-versa). This allows for a linear hydrogen bond arrangement, where the N-H and C=O groups are directly opposite each other, resulting in a stronger and more linear hydrogen bond.
-
Parallel Beta-Sheets: Here, the strands run in the same direction. This leads to a less optimal hydrogen bond geometry, with the N-H and C=O groups slightly angled, resulting in weaker hydrogen bonds. The hydrogen bonds are also somewhat skewed, and they aren't as precisely aligned as in antiparallel sheets.
Energetics of Beta-Sheet Stability: Why the Difference?
The observed greater stability of antiparallel beta-sheets stems from several key factors:
1. Hydrogen Bond Geometry: The Key Contributor
The most significant factor contributing to the stability difference is the hydrogen bond geometry. As mentioned earlier, antiparallel sheets exhibit nearly linear hydrogen bonds, leading to stronger interactions. These linear hydrogen bonds maximize the electrostatic interactions between the electronegative oxygen atom of the carbonyl group and the electropositive hydrogen atom of the amide group. In contrast, the angled hydrogen bonds in parallel sheets are weaker due to less optimal overlap of electron orbitals.
2. Steric Effects and Side-Chain Interactions: A Subtle Influence
While hydrogen bond geometry plays the dominant role, steric effects and side-chain interactions also subtly influence beta-sheet stability. The side chains in antiparallel sheets have more conformational freedom, allowing for better packing and reduced steric clashes. In parallel sheets, the side chains are often constrained, potentially leading to unfavorable interactions and decreased stability.
3. Backbone Dihedral Angles: Conformational Preferences
The backbone dihedral angles (φ and ψ) also play a role in beta-sheet stability. Antiparallel beta-sheets generally exhibit dihedral angles that are more energetically favorable compared to parallel sheets. These preferred angles contribute to the overall stability of the antiparallel arrangement.
4. Solvent Effects: Hydration and Interactions
The surrounding solvent environment also influences beta-sheet stability. The hydration patterns of antiparallel and parallel sheets differ, with antiparallel sheets often exhibiting more favorable hydration patterns. These subtle differences in hydration contribute to the overall energy difference between the two configurations.
Experimental Evidence and Computational Studies: Validating the Hypothesis
Numerous experimental studies using techniques like X-ray crystallography, nuclear magnetic resonance (NMR) spectroscopy, and circular dichroism (CD) spectroscopy have confirmed the greater prevalence and stability of antiparallel beta-sheets in naturally occurring proteins. These experiments have consistently shown that antiparallel beta-sheets are more frequently observed and often form more readily than parallel sheets.
Computational studies, such as molecular dynamics simulations and quantum mechanical calculations, have further supported this observation. These simulations allow researchers to investigate the energetics of beta-sheet formation in detail, providing insights into the factors driving the stability difference. These computational studies consistently demonstrate that antiparallel beta-sheets have lower free energy compared to their parallel counterparts, thus confirming their greater thermodynamic stability.
Exceptions and Contextual Factors: Not a Universal Rule
While antiparallel beta-sheets are generally more stable, it's crucial to remember that this is not a universal rule. Certain factors can influence the stability of beta-sheets, potentially leading to situations where parallel sheets are favored:
-
Sequence Context: The specific amino acid sequence can influence the formation and stability of beta-sheets. Certain amino acid residues may be more favorable for parallel arrangements, potentially outweighing the inherent stability advantage of antiparallel configurations. For instance, the presence of specific proline residues can influence the formation of parallel structures.
-
Protein Folding Pathway: The folding pathway of a protein can also impact the final structure. Kinetic factors, rather than purely thermodynamic considerations, might lead to the formation of a less stable parallel sheet even if an antiparallel arrangement would be thermodynamically more favorable.
-
Specific Functional Requirements: In some cases, a parallel arrangement might be required for a specific protein function, even if it is less thermodynamically stable. The functional advantage may outweigh the stability difference.
The Importance of Beta-Sheet Stability in Protein Function
The stability of beta-sheets is directly linked to the overall stability and function of proteins. Beta-sheets are crucial structural elements in many proteins, playing vital roles in various biological processes. Their stability is essential for maintaining the overall protein structure and function. Destabilization of beta-sheets can lead to protein misfolding and aggregation, which is often associated with various diseases, including Alzheimer's and Parkinson's diseases.
Conclusion: A nuanced perspective on beta-sheet stability
In summary, while antiparallel beta-sheets are generally considered more stable than parallel beta-sheets, the difference is not absolute. The greater stability of antiparallel arrangements is primarily attributed to the superior hydrogen bond geometry, with additional contributions from steric factors, dihedral angles, and solvent effects. However, sequence context, protein folding pathways, and functional requirements can influence the final structure, leading to exceptions where parallel sheets are observed. Further research continues to refine our understanding of the complex interplay of factors governing beta-sheet stability and its crucial implications for protein structure, function, and disease. Understanding this intricate relationship is essential for advancements in fields like protein engineering and drug design. The continuous exploration of this fundamental aspect of protein structure promises to reveal even more insights into the remarkable complexity of biological systems.
Latest Posts
Latest Posts
-
What Is The Least Common Multiple Of 10 And 7
Mar 04, 2025
-
How Many Chambers Does A Frog Heart Have
Mar 04, 2025
-
Do Metals Form Anions Or Cations
Mar 04, 2025
-
Least Common Multiple 15 And 9
Mar 04, 2025
-
What Are The Factors Of 29
Mar 04, 2025
Related Post
Thank you for visiting our website which covers about Are Antiparalell Beta Sheets Mrore Stable . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
