Do Metals Form Anions Or Cations
Juapaving
Mar 04, 2025 · 5 min read
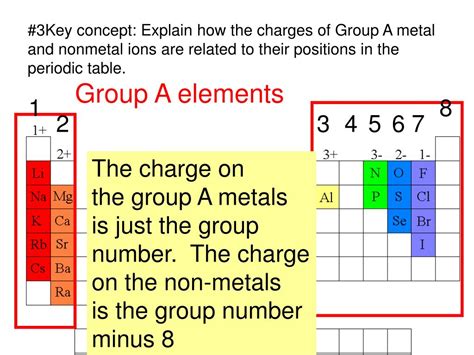
Table of Contents
Do Metals Form Anions or Cations? A Deep Dive into Metallic Bonding and Ion Formation
The question of whether metals form anions or cations is fundamental to understanding chemistry. The answer, in short, is that metals overwhelmingly form cations, positively charged ions. This characteristic stems from their electronic structure and the nature of metallic bonding. However, the nuance is far richer than this simple statement suggests, and this article will explore the intricacies of metallic ion formation, delving into the exceptions and complexities that challenge this general rule.
Understanding Ion Formation: A Review of Basic Principles
Before focusing on metals specifically, let's revisit the basic principles of ion formation. Ions are atoms or molecules that carry a net electrical charge. This charge arises from an imbalance in the number of protons (positively charged) and electrons (negatively charged). Atoms strive for a stable electron configuration, often resembling that of a noble gas (Group 18 elements). They achieve this stability through chemical bonding, including the formation of ions.
There are two main types of ions:
- Cations: Positively charged ions formed when an atom loses electrons.
- Anions: Negatively charged ions formed when an atom gains electrons.
The tendency of an atom to lose or gain electrons is determined primarily by its electronegativity – a measure of an atom's ability to attract electrons towards itself in a chemical bond. Elements with low electronegativity tend to lose electrons and form cations, while those with high electronegativity tend to gain electrons and form anions.
Why Metals Typically Form Cations
Metals are characterized by their low electronegativity and low ionization energies. This means that it requires relatively little energy to remove one or more electrons from a metal atom. Their electron configurations often feature only a few electrons in their outermost shell (valence electrons). Losing these valence electrons results in a stable, completely filled inner electron shell, mimicking the configuration of a noble gas. This stable configuration is energetically favorable.
For example, consider sodium (Na). Its electron configuration is [Ne]3s¹. Losing the single 3s electron yields a Na⁺ ion with the stable electron configuration of neon ([Ne]). This process is energetically favorable because the energy gained from achieving a stable configuration outweighs the energy required to remove the electron.
The ease with which metals lose electrons to form cations is a direct consequence of their metallic bonding. In metallic bonding, valence electrons are delocalized and shared amongst a "sea" of electrons surrounding a lattice of positively charged metal ions. This shared electron structure allows for the easy movement of electrons, contributing to the characteristic properties of metals, such as conductivity and malleability. The loss of valence electrons is, therefore, a natural part of this bonding model.
Exceptions and the Formation of Metal Anions
While the vast majority of metals form cations, there are exceptions. The formation of metal anions (or metallides) is a rare phenomenon, occurring predominantly with the heavier, post-transition metals. These metals possess a greater capacity to gain electrons due to their relatively low electronegativity values compared to typical nonmetals, and certain specific electronic configurations.
The most well-known examples involve elements like lead, tin and bismuth. These elements can exist in unusual oxidation states, and under specific conditions, can potentially show negative oxidation states, forming anions. These conditions are often unusual and require highly reducing environments.
Factors Favoring Metal Anion Formation:
- High coordination number: Metal atoms in specific crystal structures can be surrounded by a sufficient number of other atoms, allowing for the acceptance of additional electrons.
- Relatively low ionization energy: Although higher than that of alkali metals, the ionization energy of heavier post-transition metals is low enough that under highly reducing conditions, they might be induced to accept electrons.
- Strong reducing agents: The formation of metal anions necessitates the presence of extremely strong reducing agents, providing the necessary electrons for the process. This is rarely seen under standard conditions.
It's crucial to emphasize that the formation of metal anions is not a common occurrence. The energy required to overcome the strong positive charge of the metal nucleus to add more electrons is considerable. Thus, metal anions are extremely unstable and highly reactive, existing primarily in specialized environments and compounds.
Exploring the Properties of Metal Cations
The properties of metal cations are heavily influenced by their charge and size. Higher charges and smaller sizes lead to greater electrostatic interactions, resulting in several significant effects:
- Polarizing power: Highly charged, smaller cations can polarize the electron clouds of nearby anions, leading to stronger bonds and influencing the physical and chemical properties of the resulting compounds.
- Solubility: The solubility of metal salts often depends on the charge and size of the cation. Smaller, highly charged cations tend to form insoluble compounds with certain anions.
- Reactivity: The reactivity of metal cations influences their redox behavior and their participation in various chemical reactions.
Analyzing Specific Examples of Metal Cation Formation
Let's examine some specific examples to illustrate the principles discussed above:
- Alkali metals (Group 1): These metals readily lose one electron to form +1 cations (e.g., Na⁺, K⁺). Their low electronegativity and single valence electron make cation formation energetically favorable.
- Alkaline earth metals (Group 2): These metals lose two electrons to form +2 cations (e.g., Mg²⁺, Ca²⁺). Again, the low electronegativity and two valence electrons facilitate this process.
- Transition metals: Transition metals exhibit a wider range of oxidation states due to their partially filled d orbitals. They can lose various numbers of electrons, leading to cations with different charges (e.g., Fe²⁺, Fe³⁺, Cu⁺, Cu²⁺). The stability of these different oxidation states depends on factors such as ligand field stabilization.
- Post-transition metals: These metals demonstrate a greater variety in oxidation states and can potentially form anions, albeit under exceptional conditions.
Conclusion: The Predominance of Metal Cations
In conclusion, although exceptions exist, the overwhelming majority of metals form cations, not anions. This is primarily due to their low electronegativity, low ionization energies, and the energetic favorability of achieving a noble gas electron configuration by losing valence electrons. The formation of metal anions, while possible under highly specific conditions, remains a rare and often unstable phenomenon. Understanding the interplay between electronegativity, ionization energy, and electronic structure is critical to predicting the ionic behavior of metallic elements. The properties of metal cations, in turn, play a significant role in determining the characteristics of various compounds and materials. Further research into the exceptional conditions leading to metal anion formation may reveal valuable insights into the broader field of chemical bonding.
Latest Posts
Latest Posts
-
How Many Lines Of Symmetry Does A Rectangle Have
Mar 04, 2025
-
Which Of The Following Statements Is Correct
Mar 04, 2025
-
What Is The Square Root Of 121
Mar 04, 2025
-
How Many Inches Is 25 Cm
Mar 04, 2025
-
How Many Milliseconds In A Minute
Mar 04, 2025
Related Post
Thank you for visiting our website which covers about Do Metals Form Anions Or Cations . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
