Anything That Has Mass And Occupies Space Is Called
Juapaving
Mar 14, 2025 · 6 min read
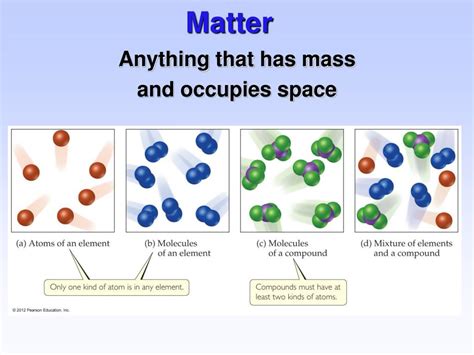
Table of Contents
Anything That Has Mass and Occupies Space Is Called Matter: A Deep Dive into the Fundamental Building Block of the Universe
Anything that has mass and occupies space is called matter. This seemingly simple definition belies the incredible complexity and richness of the concept. From the smallest subatomic particles to the largest galaxies, matter forms the foundation of everything we see, touch, and experience in the universe. Understanding matter is crucial to understanding the universe itself, and this exploration delves into its various facets, properties, and classifications.
What is Matter? A Closer Look
The fundamental definition of matter—something possessing both mass and volume—is a cornerstone of physics and chemistry. Mass refers to the amount of matter an object contains, often perceived as its weight. Volume, on the other hand, signifies the amount of three-dimensional space an object occupies. Everything from a grain of sand to a star satisfies these criteria and thus qualifies as matter.
However, the concept of matter has evolved significantly over time. Early understandings were based on macroscopic observations, but advancements in physics, particularly quantum mechanics, have revealed a far more nuanced picture. We now know that matter is composed of fundamental particles governed by intricate forces and interactions.
The Building Blocks of Matter: Atoms and Molecules
At the heart of matter lie atoms, the smallest units of an element that retain its chemical properties. These atoms consist of a dense nucleus containing protons and neutrons, surrounded by orbiting electrons. The number of protons determines the element's identity (e.g., hydrogen has one proton, oxygen has eight).
Atoms combine to form molecules, the next level of organization in matter. Water (H₂O), for instance, is a molecule composed of two hydrogen atoms and one oxygen atom. The properties of molecules, and thus the matter they form, depend on the types and arrangement of the constituent atoms. The diversity of molecules is astonishing, giving rise to the immense variety of substances we encounter in our world.
States of Matter: Solid, Liquid, Gas, and Plasma
Matter exists in different states, primarily determined by the arrangement and interaction of its constituent particles. The four fundamental states are:
-
Solid: Solids possess a fixed shape and volume. Their particles are tightly packed and exhibit strong intermolecular forces, leading to rigidity. Examples include rocks, ice, and wood.
-
Liquid: Liquids have a fixed volume but adopt the shape of their container. Their particles are less tightly packed than in solids and exhibit weaker intermolecular forces, allowing for flow. Examples include water, oil, and mercury.
-
Gas: Gases have neither a fixed shape nor a fixed volume. Their particles are widely dispersed and exhibit negligible intermolecular forces, allowing them to expand to fill any available space. Examples include air, helium, and carbon dioxide.
-
Plasma: Plasma is an ionized gas, meaning its atoms have lost or gained electrons, resulting in a mixture of positively and negatively charged particles. Plasmas are common in stars and are used in various technologies, such as fluorescent lights and plasma TVs. The behavior of plasma is highly influenced by electromagnetic fields.
Beyond these four main states, other exotic states of matter exist under extreme conditions, such as Bose-Einstein condensates and quark-gluon plasma, often studied in advanced physics research.
Properties of Matter: Physical and Chemical
Matter exhibits a wide range of properties that can be broadly categorized as physical or chemical:
Physical Properties
Physical properties are characteristics that can be observed or measured without changing the substance's chemical composition. These include:
- Density: The mass per unit volume of a substance.
- Melting point: The temperature at which a solid changes to a liquid.
- Boiling point: The temperature at which a liquid changes to a gas.
- Color: The visual appearance of a substance.
- Conductivity: The ability of a substance to conduct heat or electricity.
- Solubility: The ability of a substance to dissolve in a solvent.
- Hardness: Resistance to scratching or indentation.
- Malleability: Ability to be hammered or rolled into sheets.
- Ductility: Ability to be drawn into wires.
Chemical Properties
Chemical properties describe how a substance reacts with other substances, involving a change in its chemical composition. These include:
- Flammability: The ability of a substance to burn in the presence of oxygen.
- Reactivity: How readily a substance reacts with other substances.
- Toxicity: The degree to which a substance is poisonous.
- Acidity/Basicity: The pH of a substance, indicating its acidity or alkalinity.
- Oxidation: The ability of a substance to react with oxygen.
- Reduction: The ability of a substance to gain electrons.
Classification of Matter: Pure Substances and Mixtures
Matter can be further classified into pure substances and mixtures based on its composition:
Pure Substances
Pure substances have a fixed chemical composition and consistent properties throughout. They can be further categorized into:
- Elements: Substances composed of only one type of atom. Examples include iron (Fe), oxygen (O₂), and gold (Au).
- Compounds: Substances composed of two or more types of atoms chemically bonded together in fixed proportions. Examples include water (H₂O), salt (NaCl), and carbon dioxide (CO₂).
Mixtures
Mixtures contain two or more substances physically combined, retaining their individual properties. The composition of a mixture can vary. Mixtures are classified as:
- Homogeneous mixtures: The components are uniformly distributed throughout the mixture, appearing as a single phase. Examples include saltwater, air, and sugar dissolved in water.
- Heterogeneous mixtures: The components are not uniformly distributed, exhibiting distinct phases. Examples include sand and water, oil and water, and a salad.
The Importance of Understanding Matter
The study of matter is fundamental to numerous scientific disciplines, including chemistry, physics, materials science, and biology. Understanding the properties and behavior of matter allows us to:
- Develop new materials: By manipulating the properties of matter at the atomic and molecular levels, we can create materials with specific characteristics for diverse applications.
- Improve existing technologies: Knowledge of matter helps optimize industrial processes, improve energy efficiency, and develop more sustainable technologies.
- Understand biological systems: Living organisms are composed of matter, and understanding its behavior is critical to comprehending biological processes, disease, and developing new medicines.
- Explore the universe: The study of matter extends beyond Earth, helping us understand the composition and evolution of stars, galaxies, and the universe as a whole.
From the tiniest particles to the grandest structures, matter shapes our world. Its intricacies continue to fascinate and challenge scientists, pushing the boundaries of our knowledge and leading to innovative discoveries that shape our future. The simple definition – anything that has mass and occupies space – unlocks a vast and ever-expanding realm of scientific inquiry. Understanding this fundamental concept is vital for anyone seeking to grasp the complexities of the natural world and our place within it.
Latest Posts
Latest Posts
-
How To Calculate Abundance Of An Isotope
Mar 14, 2025
-
What Are The Prime Factors Of 85
Mar 14, 2025
-
Cell Wall And Cell Membrane Compare And Contrast
Mar 14, 2025
-
What Is The Gas Released During Photosynthesis
Mar 14, 2025
-
What Is The Prime Factorization Of 160
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about Anything That Has Mass And Occupies Space Is Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
