Another Name For A Dehydration Reaction Is A Reaction.
Juapaving
Mar 14, 2025 · 6 min read
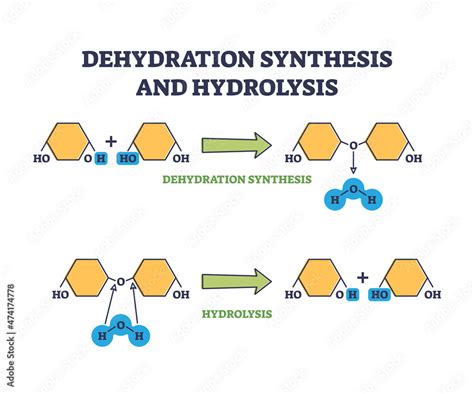
Table of Contents
Another Name for a Dehydration Reaction is a Condensation Reaction
Dehydration reactions are fundamental processes in chemistry and biology, playing crucial roles in the synthesis of numerous important molecules. Understanding their mechanism and significance is vital for grasping many biological and chemical concepts. While often referred to as dehydration reactions, they are more accurately and comprehensively termed condensation reactions. This article will delve deep into the intricacies of these reactions, explaining the nomenclature, mechanisms, examples, and biological significance.
Understanding Dehydration Reactions: The Removal of Water
A dehydration reaction, or more correctly, a condensation reaction, involves the joining of two molecules with the simultaneous removal of a water molecule (H₂O). This seemingly simple process is incredibly significant, enabling the formation of complex polymers from smaller monomers. The essence lies in the removal of a hydroxyl group (-OH) from one molecule and a hydrogen atom (-H) from another. These combine to form water, leaving the two original molecules linked together.
Key Characteristics of Dehydration/Condensation Reactions:
- Removal of Water: The defining characteristic is the loss of a water molecule. This is what distinguishes them from other reaction types.
- Formation of a Larger Molecule: Two smaller molecules combine to create a larger molecule, often a polymer.
- Requires Energy: Dehydration reactions are generally endergonic, meaning they require an input of energy to proceed. This energy is often supplied by enzymes in biological systems or by heat in laboratory settings.
- Reversible: While the forward reaction is a dehydration/condensation, the reverse reaction is a hydrolysis reaction, where water is added to break the bond and separate the molecules.
Why "Condensation Reaction" is a More Accurate Term
While "dehydration reaction" accurately describes the removal of water, the term "condensation reaction" provides a more comprehensive and descriptive name. This is because it emphasizes the process of two molecules coming together ("condensing") to form a larger molecule. The removal of water is a consequence of this condensation, not the defining feature itself. The focus shifts from the byproduct (water) to the primary process: the joining of molecules.
This more inclusive terminology is particularly useful when considering reactions that don't strictly involve the removal of a water molecule. Some condensation reactions may involve the removal of other small molecules, such as methanol or ammonia. Using "condensation reaction" prevents ambiguity and encompasses a wider range of similar processes.
Mechanisms of Condensation Reactions
The precise mechanism of a condensation reaction varies depending on the specific molecules involved, but the general principles remain consistent. The reaction often involves several steps, including:
- Approximation: The two reactant molecules must come into close proximity, allowing functional groups to interact. This step is often facilitated by enzymes in biological systems or catalysts in laboratory settings.
- Nucleophilic Attack: One molecule acts as a nucleophile (electron-rich species), attacking an electrophilic site (electron-deficient species) on the other molecule.
- Bond Formation: A new covalent bond forms between the two molecules, creating a larger molecule.
- Elimination of Water: The hydroxyl group (-OH) and a hydrogen atom (-H) are eliminated from the newly formed molecule, resulting in the formation of water.
Examples of Condensation Reactions: From Simple to Complex
Condensation reactions are ubiquitous in chemistry and biology, contributing to the formation of a vast array of compounds. Here are a few examples:
1. Esterification: Formation of Esters
Esterification is a classic example of a condensation reaction where a carboxylic acid reacts with an alcohol to form an ester and water. This is a crucial reaction in organic chemistry, finding applications in the synthesis of fragrances, flavors, and polymers.
Example: Ethanoic acid (acetic acid) reacts with ethanol to form ethyl ethanoate (ethyl acetate) and water.
2. Peptide Bond Formation: Building Proteins
The synthesis of proteins, the workhorses of biological systems, relies heavily on condensation reactions. Amino acids link together via peptide bonds to form polypeptide chains. The peptide bond is formed through a condensation reaction between the carboxyl group (-COOH) of one amino acid and the amino group (-NH₂) of another, releasing a water molecule.
Example: Glycine and alanine combine to form a dipeptide, releasing water.
3. Glycosidic Bond Formation: Constructing Carbohydrates
Carbohydrates, such as starch and cellulose, are polymers of sugar monomers (monosaccharides). The linkage between these monosaccharides is a glycosidic bond, formed via a condensation reaction. This process removes a water molecule, joining the monosaccharides into a larger carbohydrate molecule.
Example: Glucose molecules combine to form maltose, a disaccharide, with the release of water.
4. Formation of Phosphodiester Bonds in Nucleic Acids: The Building Blocks of Life
Nucleic acids, DNA and RNA, are fundamental to life, carrying genetic information. The nucleotides that make up these polymers are linked by phosphodiester bonds, which are formed via a condensation reaction. This reaction involves the dehydration between the phosphate group of one nucleotide and the hydroxyl group of another.
Example: Two nucleotides combine to form a dinucleotide, with the removal of a water molecule.
5. Synthesis of Polyesters and Polyamides: Industrial Applications
Condensation reactions are widely used in industrial settings for the synthesis of polymers such as polyesters and polyamides (nylons). These reactions involve the joining of monomers with the release of small molecules, creating long chains that have various applications, from clothing to packaging.
Biological Significance of Condensation Reactions
The significance of condensation reactions in biological systems cannot be overstated. They are essential for:
- Protein Synthesis: The synthesis of proteins, vital for all biological processes, is entirely dependent on condensation reactions.
- Carbohydrate Metabolism: Condensation reactions play a role in the synthesis and breakdown of carbohydrates, providing energy and structural components.
- Nucleic Acid Replication: DNA and RNA replication rely on condensation reactions for the formation of phosphodiester bonds, ensuring faithful transmission of genetic information.
- Lipid Biosynthesis: The formation of complex lipids, such as triglycerides and phospholipids, also involves condensation reactions.
- Enzyme Catalysis: Enzymes catalyze many condensation reactions, making them highly efficient and specific.
The Reverse Reaction: Hydrolysis
It's crucial to remember that condensation reactions are reversible. The reverse process, called hydrolysis, involves the addition of water to break the bond between two molecules. This is also a crucial process in biological systems, allowing for the breakdown of large polymers into their constituent monomers. For instance, the digestion of proteins involves hydrolysis of peptide bonds, breaking down proteins into individual amino acids. Similarly, the digestion of carbohydrates involves hydrolysis of glycosidic bonds, releasing simple sugars.
Conclusion: A Fundamental Process in Chemistry and Biology
Dehydration reactions, more accurately termed condensation reactions, are fundamental processes underpinning many aspects of chemistry and biology. They are responsible for the synthesis of a vast array of molecules, from simple esters to complex biological polymers like proteins and nucleic acids. Understanding their mechanisms and significance provides a deeper appreciation of the intricate processes that govern life and the synthetic capabilities of chemistry. While the term "dehydration reaction" is commonly used, appreciating the broader context of "condensation reaction" allows for a more complete and accurate understanding of these crucial reactions and their extensive impact on the molecular world. The reversible nature of these reactions, with hydrolysis being the reverse process, completes the picture of their importance in dynamic biological systems and industrial applications.
Latest Posts
Related Post
Thank you for visiting our website which covers about Another Name For A Dehydration Reaction Is A Reaction. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
