Is Tap Water A Mixture Or Pure Substance
Juapaving
Mar 11, 2025 · 5 min read
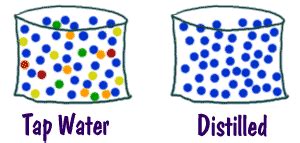
Table of Contents
Is Tap Water a Mixture or a Pure Substance? A Deep Dive into the Composition of Your Everyday Drink
The simple question, "Is tap water a pure substance or a mixture?" leads us down a fascinating path exploring the chemical composition of everyday life. The answer, while seemingly straightforward, reveals a complex world of dissolved minerals, gases, and even potential contaminants. This article will delve deep into the science behind tap water, examining its components and why it's definitively classified as a mixture.
Understanding Pure Substances and Mixtures
Before we dissect the complexities of tap water, let's establish a clear understanding of the fundamental differences between pure substances and mixtures.
Pure Substances: The Building Blocks
A pure substance has a fixed chemical composition throughout. This means it's made up of only one type of atom or molecule. Examples include:
- Elements: These are substances made up of only one type of atom, like oxygen (O₂), hydrogen (H₂), or gold (Au).
- Compounds: These are substances formed by the chemical combination of two or more elements in fixed proportions, like water (H₂O) or table salt (NaCl). A compound always has the same ratio of elements.
Mixtures: A Blend of Substances
Mixtures, on the other hand, are composed of two or more substances that are physically combined, but not chemically bonded. The components retain their individual properties and can be separated using physical methods like filtration or distillation. Mixtures can be:
- Homogeneous: The components are evenly distributed throughout the mixture, making it appear uniform. Examples include saltwater, air, and (surprisingly!) tap water.
- Heterogeneous: The components are not evenly distributed, and different parts of the mixture have different properties. Examples include sand and water, or a salad.
Deconstructing Tap Water: A Complex Mixture
Now, let's turn our attention to tap water. While the chemical formula H₂O accurately represents the primary component – water – tap water is far from pure H₂O. The journey of water from its source to your tap introduces a multitude of substances, making it a complex mixture.
The Source: Natural Variations
The initial composition of water depends largely on its source. Groundwater, drawn from aquifers, may contain dissolved minerals like calcium, magnesium, and iron, picked up as water percolates through soil and rock formations. Surface water, from rivers and lakes, can have higher levels of organic matter and suspended sediments.
Treatment Processes: Adding and Removing Substances
Water treatment plants employ several processes to ensure tap water is safe for consumption. These processes can add or remove substances, further altering its composition. Common treatment steps include:
- Coagulation and Flocculation: Removing suspended solids by adding chemicals that cause particles to clump together.
- Sedimentation: Allowing heavier particles to settle out of the water.
- Filtration: Passing water through various filter media to remove smaller particles and impurities.
- Disinfection: Killing harmful bacteria and viruses using chlorine, chloramine, or UV light.
- Fluoridation: Adding fluoride to improve dental health (this is not universally practiced).
These processes aim to remove harmful contaminants while potentially adding other substances. Therefore, the final composition of tap water is a dynamic blend influenced by its source, the treatment process, and the distribution system.
Common Components of Tap Water: Beyond H₂O
Tap water is a heterogeneous mixture containing a variety of dissolved substances, including:
-
Dissolved Minerals: Calcium, magnesium, sodium, potassium, and others are commonly found in varying concentrations depending on the water source and treatment. These minerals contribute to the water's hardness. Hard water can leave mineral deposits on appliances and fixtures.
-
Dissolved Gases: Oxygen, nitrogen, and carbon dioxide are naturally present in water. The levels can fluctuate based on the water's exposure to the atmosphere.
-
Disinfectants: Chlorine or chloramine, added to kill harmful microorganisms, remain in the water at low concentrations. These byproducts can contribute to taste and odor, and some people prefer to use a filter to reduce their levels.
-
Trace Contaminants: Despite rigorous treatment, small amounts of other substances might persist, including heavy metals (though typically at levels well below safety standards), pesticides, pharmaceuticals, and industrial byproducts. The concentration of these contaminants varies considerably depending on geographic location and industrial activity.
Why Tap Water is a Mixture: Evidence and Examples
Several lines of evidence firmly establish tap water as a mixture:
-
Variable Composition: The composition of tap water is not fixed. It varies depending on the water source, treatment methods, and even the time of year. This variability is a hallmark of mixtures.
-
Separation of Components: Individual components of tap water can be separated using various physical methods. Distillation, for example, can separate water from dissolved solids. Filtration removes suspended particles. These separation techniques are impossible with pure substances.
-
Retention of Individual Properties: The components of tap water retain their individual characteristics. Dissolved minerals contribute to hardness, while disinfectants impact taste and odor. These individual properties would be lost if the components were chemically bonded.
-
Observational Evidence: The presence of suspended particles, cloudiness (turbidity), or varying levels of taste and odor visually demonstrates the presence of multiple substances—another characteristic of mixtures.
The Importance of Understanding Tap Water's Composition
Recognizing tap water as a mixture rather than a pure substance is crucial for several reasons:
-
Public Health: Understanding the potential presence of contaminants in tap water informs public health regulations and treatment strategies to ensure safe drinking water.
-
Environmental Protection: Analyzing the composition of tap water helps monitor the impact of human activities on water quality and guide environmental protection efforts.
-
Water Treatment Optimization: Knowing the specific components of tap water allows for the optimization of water treatment processes to effectively remove contaminants and ensure the water meets quality standards.
-
Consumer Choice: Understanding the components allows consumers to make informed choices about water filtration or treatment systems, if desired, to further purify their water based on their individual concerns.
Conclusion: A Complex but Essential Mixture
The seemingly simple question of whether tap water is a pure substance or a mixture leads to a rich exploration of the complexities of water chemistry and the crucial role of water treatment in public health. The evidence overwhelmingly supports the classification of tap water as a complex mixture, a dynamic blend of H₂O and a myriad of dissolved and suspended substances. Understanding this composition empowers individuals and policymakers to make informed decisions regarding water quality, treatment, and consumption. It underscores the importance of ongoing monitoring and regulation to ensure the safe and reliable provision of this essential resource.
Latest Posts
Latest Posts
-
What Is 14 Centimeters In Inches
May 09, 2025
-
Label A Label B Label C Label D
May 09, 2025
-
Why Hydrogen Is In Group 1
May 09, 2025
-
How Many Mins In 9 Hours
May 09, 2025
-
What Is The Percentage Of 0 8
May 09, 2025
Related Post
Thank you for visiting our website which covers about Is Tap Water A Mixture Or Pure Substance . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.