A Substance Formed In A Chemical Reaction
Juapaving
Mar 20, 2025 · 6 min read
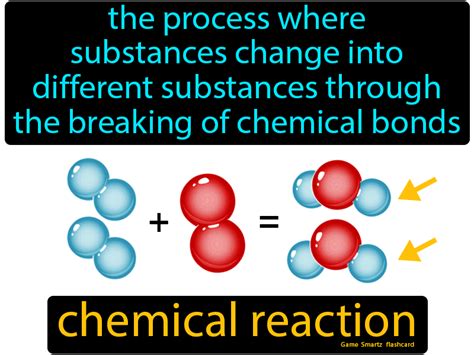
Table of Contents
A Substance Formed in a Chemical Reaction: Understanding Products and Their Significance
Chemical reactions are the fundamental processes that govern the transformation of matter. They involve the rearrangement of atoms and molecules, leading to the formation of new substances with distinct properties. Understanding the products of these reactions – the substances formed – is crucial in various scientific fields, from industrial chemistry to biology and environmental science. This article delves deep into the nature of products formed in chemical reactions, exploring their characteristics, identification, and significance across different disciplines.
What are Products in a Chemical Reaction?
A chemical reaction can be simply represented by the equation: Reactants → Products. Reactants are the starting materials, the substances that undergo transformation. Products, on the other hand, are the new substances formed as a result of the chemical changes occurring between the reactants. The formation of products marks the completion of a chemical reaction. These products possess different physical and chemical properties compared to the original reactants. For example, in the reaction between hydrogen and oxygen to form water, hydrogen and oxygen are the reactants, and water is the product.
Key Characteristics of Products:
- Different Properties: Products invariably exhibit different properties compared to their reactant counterparts. This difference can manifest in various aspects:
- Physical Properties: Changes in color, odor, melting point, boiling point, density, and solubility are common observations.
- Chemical Properties: The chemical reactivity of the product often differs significantly from the reactants. For instance, the product might be less reactive, more reactive, or exhibit entirely new reactive tendencies.
- Conservation of Mass: A fundamental principle governing chemical reactions is the conservation of mass. This implies that the total mass of the reactants equals the total mass of the products. No mass is lost or gained during a chemical reaction, only rearranged.
- Stoichiometry: The quantitative relationship between reactants and products is defined by stoichiometry. This aspect involves the molar ratios in which reactants combine and the corresponding amounts of products formed. Understanding stoichiometry is crucial for accurately predicting the yield of a reaction.
- State of Matter: Products can exist in different states of matter—solid, liquid, or gas—depending on the reaction conditions and the chemical nature of the product. Phase changes during a reaction are often indicative of product formation.
Identifying Products in Chemical Reactions:
Identifying the products of a chemical reaction requires a combination of observational techniques and analytical methods.
Qualitative Observations:
- Visual Inspection: Observing changes in color, the formation of precipitates (solids), the evolution of gases (bubbles), or changes in temperature can provide initial clues about the products formed.
- Odor Detection: Some reactions produce products with distinct odors, aiding in their identification. However, caution must be exercised as some gases can be toxic.
Quantitative Analysis:
- Titration: This technique involves reacting a known volume of a solution with a solution of known concentration to determine the concentration of an unknown solution, often providing information about product quantities.
- Spectroscopy: Various spectroscopic techniques, such as UV-Vis spectroscopy, infrared (IR) spectroscopy, and nuclear magnetic resonance (NMR) spectroscopy, are employed to identify the structure and composition of products based on their interaction with electromagnetic radiation.
- Chromatography: Techniques like gas chromatography (GC) and high-performance liquid chromatography (HPLC) separate the components of a mixture, allowing for the identification and quantification of individual products.
- Mass Spectrometry: This technique determines the mass-to-charge ratio of ions, providing information about the molecular weight and elemental composition of products.
Significance of Products in Various Fields:
The formation of specific products in chemical reactions has far-reaching implications across diverse scientific and technological domains.
Industrial Chemistry:
The entire chemical industry revolves around the controlled production of specific products through chemical reactions. Examples include:
- Polymer Synthesis: The production of plastics, synthetic fibers, and rubbers involves the polymerization of monomers – small molecules reacting to form large macromolecules.
- Pharmaceutical Production: The synthesis of pharmaceuticals relies on intricate multi-step chemical reactions to create molecules with specific therapeutic effects. Precise control over the products formed is critical in drug manufacturing.
- Fertilizer Production: The Haber-Bosch process, a crucial industrial reaction, produces ammonia, a key component of fertilizers, by reacting nitrogen and hydrogen. The efficiency of this process is crucial for global food production.
- Material Science: Advanced materials, such as high-strength alloys, semiconductors, and superconductors, are often synthesized through carefully controlled chemical reactions, resulting in products with tailored properties.
Environmental Science:
Understanding the products of chemical reactions is crucial for addressing environmental challenges:
- Pollution Control: Monitoring the products of combustion reactions, industrial emissions, and other pollution sources is vital for assessing environmental impact and developing effective pollution control strategies.
- Waste Management: The chemical transformation of waste materials into less harmful products is a crucial aspect of waste management and resource recovery.
- Climate Change Mitigation: Research into reactions involving greenhouse gases and their transformation into less harmful products is crucial for mitigating climate change.
Biology and Medicine:
Chemical reactions are the foundation of all biological processes. Understanding the products of these reactions is essential in various aspects of biology and medicine:
- Metabolism: Metabolic processes involve a complex network of enzyme-catalyzed reactions, resulting in the formation of various products, including energy-carrying molecules (ATP) and essential biomolecules.
- Drug Action: The effectiveness of drugs depends on their interaction with biological molecules, leading to the formation of specific products and altering biological pathways.
- Diagnostics: Many diagnostic tests rely on chemical reactions to detect specific substances or changes in biological samples.
Factors Influencing Product Formation:
Several factors influence the types and amounts of products formed in chemical reactions:
- Reactant Concentration: The concentration of reactants significantly influences reaction rates and product yields. Higher concentrations generally lead to faster reactions and higher yields, up to a certain point.
- Temperature: Temperature affects the kinetic energy of reactant molecules, influencing the reaction rate. Higher temperatures generally increase reaction rates but can also lead to side reactions and the formation of unwanted products.
- Pressure: Pressure primarily influences reactions involving gases. Increased pressure generally favors reactions that lead to a decrease in the number of gas molecules.
- Catalyst: A catalyst speeds up a reaction without being consumed itself. Catalysts can influence the product distribution, favoring the formation of desired products and suppressing the formation of unwanted byproducts.
- Solvent: The solvent used in a reaction can significantly influence reaction rates and product yields by affecting the solubility of reactants and products, and influencing interactions between molecules.
Conclusion:
The products of chemical reactions are not merely the end result of a transformation; they are the building blocks of materials, the key players in biological processes, and the subjects of intense scientific study. Understanding the formation, identification, and properties of these products is fundamental to advancing our knowledge in various scientific and technological fields. From designing new materials to developing life-saving medications and addressing environmental challenges, our ability to control and manipulate chemical reactions to produce specific products remains a driving force in human progress. The ongoing development and refinement of analytical techniques will continue to enhance our ability to understand and control the intricate world of chemical reactions and their resulting products.
Latest Posts
Latest Posts
-
What Is The Square Root Of 576
Mar 20, 2025
-
What Has 7 Sides That Is A Polygon
Mar 20, 2025
-
Is 89 Prime Or Composite Number
Mar 20, 2025
-
Where Do The Dark Reactions Occur
Mar 20, 2025
-
The Function Of The Dartos And Cremaster Muscles Is To
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about A Substance Formed In A Chemical Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
