A Solution In Which More Solute Can Be Dissolved
Juapaving
Apr 06, 2025 · 6 min read
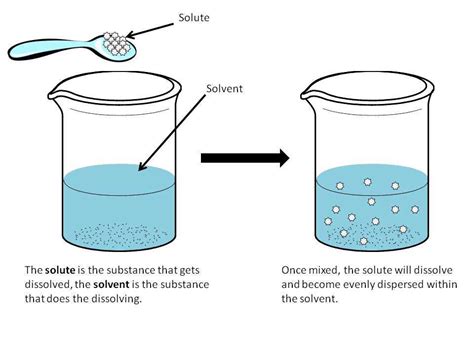
Table of Contents
A Deep Dive into Supersaturation: When More Solute Dissolves Than Expected
Solubility, the ability of a substance (the solute) to dissolve in a solvent to form a homogeneous mixture (a solution), is a fundamental concept in chemistry. We often learn about solubility limits – the maximum amount of solute that can dissolve in a given amount of solvent at a specific temperature and pressure. However, there's a fascinating phenomenon that transcends this limit: supersaturation. This article explores supersaturation, explaining what it is, how it's achieved, its applications, and its implications across various scientific fields.
Understanding Solubility and Saturation
Before delving into supersaturation, let's solidify our understanding of basic solubility principles. When a solute is added to a solvent, it begins to dissolve. As more solute is added, the rate of dissolution may initially exceed the rate of precipitation (the solute coming out of solution). Eventually, a point is reached where the rate of dissolution equals the rate of precipitation. This point is called saturation, and the resulting solution is a saturated solution. At this point, no more solute can dissolve under the given conditions. Any additional solute added will simply remain undissolved at the bottom of the container.
The solubility of a solute is often expressed in terms of its solubility limit, which can be affected by various factors:
- Temperature: The solubility of most solids in liquids increases with increasing temperature. However, this isn't always the case; some substances exhibit decreased solubility with increased temperature. Gases, on the other hand, generally become less soluble as temperature rises.
- Pressure: Pressure significantly affects the solubility of gases in liquids. Increasing pressure increases the solubility of a gas. The effect of pressure on the solubility of solids is generally negligible.
- Nature of the solute and solvent: The chemical nature of both the solute and the solvent plays a crucial role in determining solubility. "Like dissolves like" is a common rule of thumb – polar solvents tend to dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes.
Entering the Realm of Supersaturation: A Solution Beyond the Limit
Supersaturation is a state where a solution contains more solute than it can theoretically hold at a given temperature and pressure. It's a metastable state, meaning it's thermodynamically unstable; the slightest perturbation can trigger the excess solute to precipitate out, returning the solution to its saturated state. Imagine a tightly packed room – you've squeezed in more people than it's designed to hold. It's unstable; any small movement can cause a domino effect. Similarly, in a supersaturated solution, the excess solute is "uncomfortably crammed" and ready to come out at the slightest provocation.
How is Supersaturation Achieved?
Several methods can be employed to create a supersaturated solution:
- Cooling a saturated solution: This is a common technique. A saturated solution is prepared at a higher temperature, where solubility is typically greater. As the solution cools slowly and carefully, the solute may remain dissolved even though its solubility at the lower temperature is less. Any disturbance, such as jarring the container or introducing a seed crystal, can initiate crystallization.
- Evaporation: Slowly evaporating the solvent from a saturated solution can also lead to supersaturation. As the solvent evaporates, the concentration of the solute increases, surpassing the solubility limit at the given temperature.
- Adding more solute: This method requires careful control and often involves adding the solute very slowly and gently to a pre-existing saturated solution while maintaining specific conditions (like temperature). A slight increase in concentration beyond the equilibrium point can result in supersaturation.
- Chemical reactions: Supersaturation can also be achieved through specific chemical reactions that produce a soluble product in excess of its solubility limit.
The Importance of Seed Crystals and Nucleation
The stability of a supersaturated solution is fragile. The process of crystallization, where the excess solute precipitates out of solution, is initiated by nucleation. This is the formation of tiny solid particles (nuclei) onto which more solute molecules can attach, leading to the growth of larger crystals. These nuclei can be formed spontaneously (homogeneous nucleation) or by introducing a small crystal of the solute (heterogeneous nucleation), also known as a seed crystal.
Seed crystals provide a ready-made surface for the solute molecules to attach to, making crystallization much more efficient. Without seed crystals, the solution may remain supersaturated for a prolonged period, but eventually, spontaneous nucleation will occur, often resulting in rapid and uncontrolled crystallization.
Applications of Supersaturation
Supersaturation finds numerous applications across diverse fields:
1. Crystallization and Material Science:
Supersaturation is a cornerstone of crystal growth techniques. By carefully controlling the supersaturation level and the nucleation process, researchers can grow high-quality crystals with specific sizes, shapes, and properties. This is crucial in various applications, including the production of:
- Semiconductors: High-purity crystals are essential for electronic devices.
- Pharmaceuticals: Drug crystals need to have specific properties for optimal bioavailability and stability.
- Optical materials: Crystals used in lasers and other optical devices require precise control over their size and quality.
2. Food Science:
Supersaturation plays a significant role in the texture and stability of certain foods:
- Candy making: The process of creating candies often involves creating supersaturated sugar solutions. Controlling the cooling rate and nucleation allows for the production of various candy textures, from hard candies to chewy caramels.
- Jam and jelly making: The high sugar concentration in jams and jellies results from a supersaturated solution, which contributes to their preservation.
3. Environmental Science:
Understanding supersaturation is essential for comprehending certain environmental phenomena, including:
- Atmospheric science: Supersaturation can occur in clouds, leading to the formation of ice crystals or precipitation.
- Oceanography: The solubility of gases in the ocean can be influenced by supersaturation.
4. Medical Applications:
Supersaturation is explored in various medical applications:
- Drug delivery systems: Supersaturated solutions can be utilized to improve the solubility and bioavailability of poorly soluble drugs.
- Biomineralization: The formation of bones and teeth involves processes similar to crystallization from supersaturated solutions.
Challenges and Considerations
While supersaturation is useful, it presents some challenges:
- Metastability: The inherent instability of supersaturated solutions can lead to unpredictable crystallization events, which can be problematic in industrial processes.
- Control and reproducibility: Achieving and maintaining a desired level of supersaturation requires precise control over various parameters, such as temperature, pressure, and the addition rate of solute.
- Crystallization kinetics: The rate of crystallization in a supersaturated solution can be difficult to predict and control.
Conclusion: A Powerful Phenomenon with Wide-Reaching Implications
Supersaturation, the ability to dissolve more solute than theoretically possible, is a captivating phenomenon with broad implications across diverse fields. Its understanding is crucial for controlling crystal growth, developing new materials, and understanding natural processes. While the metastable nature of supersaturated solutions presents challenges, mastering the techniques involved in their creation and manipulation unlocks significant opportunities for advancements in science, technology, and industry. The continued exploration of supersaturation will undoubtedly lead to further innovations and discoveries in the years to come. From the intricate structures of crystalline materials to the delightful textures of candies, the principle of exceeding solubility limits consistently surprises and inspires us.
Latest Posts
Latest Posts
-
Capacity Of Doing Work Is Called
Apr 07, 2025
-
Is Ascending Order A To Z
Apr 07, 2025
-
Which Number Is A Factor Of 51
Apr 07, 2025
-
Finding The Area Under The Curve Calculator
Apr 07, 2025
-
What Are The Factors Of 216
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about A Solution In Which More Solute Can Be Dissolved . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
