A Positively Charged Particle In An Atom Is The
Juapaving
Mar 22, 2025 · 6 min read
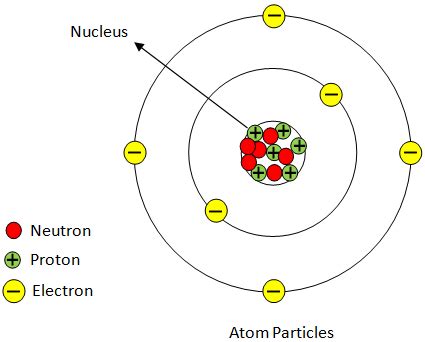
Table of Contents
A Positively Charged Particle in an Atom is the Proton: Exploring its Properties and Significance
A positively charged particle in an atom is the proton. This seemingly simple statement underpins a vast understanding of atomic structure, chemical bonding, and the behavior of matter itself. This article delves deep into the properties of protons, their role in the atom, and their broader significance in physics and chemistry.
What is a Proton?
A proton is a subatomic particle, meaning it's a fundamental constituent of atoms. It carries a single unit of positive electric charge (+1e), where 'e' represents the elementary charge. Crucially, the proton's mass is significantly larger than that of an electron, approximately 1836 times greater. This mass difference has profound implications for atomic structure and behavior. Protons are considered fermions, meaning they obey the Pauli Exclusion Principle, which dictates that no two protons can occupy the same quantum state simultaneously. This principle is vital in understanding the arrangement of protons within an atom's nucleus.
Location and Role in the Atom
Protons reside within the atom's nucleus, a tiny, dense region at the atom's center. The nucleus also houses neutrons, which are electrically neutral particles. The number of protons in an atom's nucleus, known as its atomic number, defines the element. For example, an atom with one proton is hydrogen, an atom with two protons is helium, and so on. This is fundamental to the organization of the periodic table. The positive charge of the protons is balanced by the negative charge of the electrons orbiting the nucleus, resulting in an electrically neutral atom.
Properties of Protons
Beyond their charge and mass, protons possess several other key properties:
-
Spin: Protons, like electrons, possess an intrinsic angular momentum called spin, which is quantized and contributes to their magnetic moment. This spin is often represented as ½, indicating it's a fermion.
-
Magnetic Moment: Due to their spin and charge, protons possess a magnetic moment, making them interact with magnetic fields. Nuclear magnetic resonance (NMR) spectroscopy exploits this property to analyze molecular structures.
-
Composition: Protons are not fundamental particles in the same way as electrons; they are composed of even smaller particles called quarks. Specifically, a proton comprises two up quarks and one down quark, held together by the strong nuclear force.
-
Stability: Protons are remarkably stable particles. While theoretically they can decay, the predicted half-life is vastly longer than the age of the universe, making them effectively stable for all practical purposes. This stability is critical to the stability of matter.
The Strong Nuclear Force and Proton-Proton Interaction
The protons within the nucleus are incredibly close together. Given their like charges, one might expect them to repel each other due to electrostatic forces. However, the nucleus remains stable due to the strong nuclear force, a fundamental force much stronger than electromagnetism at short ranges. This force overcomes the electrostatic repulsion between protons, binding them together within the nucleus.
The strong nuclear force is mediated by particles called gluons, which are responsible for holding the quarks within protons and also binding protons and neutrons together. The interplay between the strong nuclear force and the electromagnetic force determines the stability and structure of atomic nuclei. Understanding this intricate balance is crucial in nuclear physics and the study of radioactivity.
Isotopes and Proton Number
While the number of protons determines the element, the number of neutrons can vary. Atoms with the same number of protons but different numbers of neutrons are called isotopes. Many elements have several naturally occurring isotopes, each with slightly different properties. Some isotopes are stable, while others are radioactive and undergo decay. The study of isotopes has numerous applications, including radiometric dating and medical imaging.
The Role of Protons in Chemical Reactions
The number of protons determines an element's chemical properties. The outermost electrons, influenced by the nuclear charge, are responsible for chemical bonding and reactivity. The positive charge of the protons attracts electrons, creating the electrostatic forces that hold molecules together through ionic, covalent, and metallic bonds.
Proton-Electron Interaction and Atomic Structure
The electrostatic attraction between the positively charged protons and the negatively charged electrons is the driving force behind atomic structure. Electrons are arranged in orbitals around the nucleus, occupying specific energy levels defined by quantum mechanics. The number of protons dictates how many electrons an atom can hold, influencing its size, reactivity, and other characteristics.
Understanding this electron-proton interaction is critical to comprehend various phenomena:
-
Ionization: The removal or addition of electrons from a neutral atom creates ions, which are charged particles. This process is fundamental in many chemical reactions and technological applications.
-
Chemical Bonding: The sharing or transfer of electrons between atoms due to the proton's influence leads to the formation of chemical bonds, linking atoms into molecules. The nature of these bonds directly relates to the chemical and physical properties of substances.
-
Spectroscopy: The interaction of light with electrons can cause transitions between energy levels. This interaction, influenced by the number and arrangement of protons, is utilized in various spectroscopic techniques, allowing scientists to determine the composition and structure of matter.
Protons in Astrophysics and Cosmology
Protons are not just confined to atoms; they play a significant role in the universe at large. The majority of the visible matter in the universe is composed of protons, mostly as hydrogen nuclei (single protons). The formation of stars and galaxies depends on the gravitational collapse of vast clouds of hydrogen gas, driven by the interactions of protons and other particles. Nuclear fusion processes within stars convert protons into helium nuclei, releasing vast amounts of energy that powers stars.
The study of protons in astrophysics and cosmology provides insights into:
-
Stellar Nucleosynthesis: The process by which stars create heavier elements from hydrogen and helium through nuclear fusion reactions involving protons.
-
Cosmic Rays: High-energy protons that bombard the Earth from outer space. These particles can provide information about processes occurring in distant galaxies.
-
Big Bang Nucleosynthesis: The creation of light elements, including protons, during the early stages of the universe's evolution.
Advanced Concepts and Research
Recent research continually expands our understanding of protons. These studies involve sophisticated experimental techniques and theoretical models that delve into the intricacies of:
-
Proton Structure: Exploring the internal structure of protons and the dynamics of quarks and gluons. This involves high-energy particle collisions and advanced theoretical calculations.
-
Proton Decay: Though extremely rare, the potential decay of protons is an active area of research, with implications for particle physics and cosmology. Observing proton decay would revolutionize our understanding of fundamental particles and forces.
-
Proton Spin Crisis: The contributions of quarks and gluons to the total spin of the proton are a subject of ongoing research. The observed spin doesn’t simply add up to the expected value, presenting a puzzle for physicists.
Conclusion
The seemingly simple positively charged particle in an atom, the proton, is a fundamental building block of matter, playing a critical role in atomic structure, chemical reactions, and the universe itself. Its properties, interactions, and ongoing research continue to challenge and inspire scientists, leading to new discoveries and deepening our understanding of the physical world. From the basic principles of chemistry to the grand scale of astrophysics, the proton’s influence is undeniable and continues to be a subject of intense scientific exploration. The more we understand the proton, the more we understand the universe around us.
Latest Posts
Latest Posts
-
Write Number In Two Other Forms
Mar 22, 2025
-
What Are The Monomers Of A Dna Molecule
Mar 22, 2025
-
Two Ivory Balls Are Placed Together At Rest
Mar 22, 2025
-
Is Evaporation Of Water Endothermic Or Exothermic
Mar 22, 2025
-
Moment Of Inertia Of A Right Triangle
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about A Positively Charged Particle In An Atom Is The . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
