Why Is Sigma Bond Stronger Than Pi Bond
Juapaving
Apr 07, 2025 · 6 min read
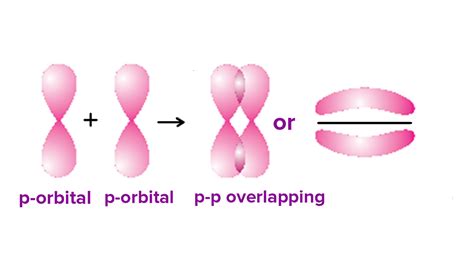
Table of Contents
Why is a Sigma Bond Stronger Than a Pi Bond? A Deep Dive into Chemical Bonding
Understanding the differences in bond strength between sigma (σ) and pi (π) bonds is fundamental to comprehending molecular structure and reactivity in chemistry. While both are covalent bonds formed by the overlap of atomic orbitals, their geometry and electron density distribution lead to significant differences in their bond strengths. This article will delve into the intricacies of sigma and pi bonds, explaining why sigma bonds consistently exhibit greater strength than their pi bond counterparts.
The Nature of Sigma (σ) Bonds
Sigma bonds are the strongest type of covalent bond. They are formed by the head-on overlap of atomic orbitals, resulting in a symmetrical electron density distribution along the internuclear axis connecting the two bonded atoms. This direct, maximal overlap of electron clouds leads to a high degree of electron sharing and strong electrostatic attraction between the positively charged nuclei and the negatively charged electron cloud.
Characteristics of Sigma Bonds:
- Head-on overlap: Atomic orbitals directly overlap along the internuclear axis.
- Cylindrical symmetry: The electron density is distributed symmetrically around the bond axis, resembling a cylinder.
- Free rotation: Sigma bonds allow free rotation around the bond axis without affecting the bond strength. This is because the overlap is maintained regardless of the rotational orientation.
- Strongest covalent bond: Due to the maximal overlap, sigma bonds are stronger than pi bonds.
- Single bonds are always sigma bonds: In a single bond between two atoms, it’s always a sigma bond.
The Nature of Pi (π) Bonds
Pi bonds, unlike sigma bonds, are formed by the sideways or lateral overlap of atomic orbitals. This overlap occurs above and below the internuclear axis, resulting in an electron density distribution concentrated above and below the plane of the bonded atoms. The electron cloud in a pi bond is not as concentrated along the internuclear axis as in a sigma bond.
Characteristics of Pi Bonds:
- Sideways overlap: Atomic orbitals overlap laterally, above and below the internuclear axis.
- Planar symmetry: The electron density is concentrated above and below the plane of the bonded atoms.
- Restricted rotation: Pi bonds restrict rotation around the bond axis. Rotation would disrupt the sideways overlap, breaking the pi bond.
- Weaker than sigma bonds: Because of the less efficient overlap compared to sigma bonds, pi bonds are weaker.
- Multiple bonds contain pi bonds: Pi bonds are only found in double and triple bonds. Double bonds consist of one sigma bond and one pi bond; triple bonds have one sigma bond and two pi bonds.
Why Sigma Bonds are Stronger: A Comparative Analysis
The key to understanding the strength difference lies in the degree of orbital overlap. Sigma bonds, with their head-on overlap, achieve maximum orbital overlap. This results in a high concentration of electron density directly between the nuclei of the bonded atoms. This strong concentration of negative charge effectively shields the positive charges of the nuclei, leading to a powerful electrostatic attraction and a strong bond.
Pi bonds, on the other hand, exhibit less effective orbital overlap. The sideways interaction leads to a more diffuse electron cloud, with the electron density concentrated above and below the internuclear axis, not directly between the nuclei. This results in a weaker electrostatic attraction between the nuclei and the electrons compared to a sigma bond.
Visualizing the Difference:
Imagine two balloons representing atomic orbitals. In a sigma bond, the balloons are pressed together head-on, resulting in a significant area of overlap. In contrast, with a pi bond, the balloons are pressed together sideways. While there is overlap, it's significantly less than in the head-on overlap, leading to a smaller shared volume and a weaker attraction.
Quantifying the Difference:
While the exact strength of a bond depends on the specific atoms involved, the general trend is consistent across various molecules. The bond energy (the energy required to break a bond) provides a quantitative measure of bond strength. Sigma bonds consistently exhibit higher bond energies than pi bonds. For example, in ethene (C₂H₄), the C=C double bond comprises one sigma bond and one pi bond. The sigma bond is significantly stronger than the pi bond. Breaking the pi bond requires less energy than breaking the sigma bond.
Bond Order and Bond Strength
The bond order is another crucial factor influencing bond strength. Bond order refers to the number of chemical bonds between a pair of atoms. A single bond has a bond order of 1 (one sigma bond), a double bond has a bond order of 2 (one sigma bond and one pi bond), and a triple bond has a bond order of 3 (one sigma bond and two pi bonds).
While a higher bond order generally correlates with increased bond strength, it's essential to understand the contribution of sigma and pi bonds. Even though a triple bond is stronger than a double bond, which is stronger than a single bond, the sigma bond component plays the most critical role in determining the overall bond strength in each case. The additional pi bonds in double and triple bonds provide additional strength, but not to the same extent as the sigma bond.
Implications for Molecular Geometry and Reactivity
The difference in sigma and pi bond strengths has significant implications for the shape and reactivity of molecules. The stronger sigma bonds form the backbone of molecular structures, dictating the basic framework of a molecule. The presence and location of pi bonds influence the molecule's overall geometry and its reactivity. For example, the presence of pi bonds makes molecules more susceptible to addition reactions, due to the relative ease of breaking the pi bonds.
Examples:
- Ethene (C₂H₄): The planar geometry of ethene is a direct consequence of the pi bond. Rotation around the C=C bond is restricted due to the presence of the pi bond, maintaining the planar structure.
- Benzene (C₆H₆): The delocalized pi system in benzene contributes significantly to its stability and unique reactivity. The pi bonds are distributed evenly throughout the ring.
- Alkynes: Alkynes contain a triple bond (one sigma and two pi bonds). The strong sigma bond forms the foundation of the linear geometry, while the pi bonds influence the reactivity of alkynes through addition reactions.
Conclusion: Sigma Bonds Reign Supreme
In summary, sigma bonds are stronger than pi bonds primarily due to their more effective head-on orbital overlap. This leads to a greater electron density concentration between the bonded atoms, resulting in stronger electrostatic attraction and higher bond energy. While pi bonds contribute to overall bond strength in multiple bonds, they do not reach the strength of a sigma bond due to their less efficient sideways overlap. Understanding this fundamental difference is crucial for comprehending molecular structure, stability, and reactivity in organic and inorganic chemistry. The dominance of the sigma bond forms the bedrock of our understanding of chemical bonding and molecular properties.
Latest Posts
Latest Posts
-
Is Salad A Mixture Or Solution
Apr 09, 2025
-
Which Of The Earths Layers Is The Thinnest
Apr 09, 2025
-
X 1 On A Number Line
Apr 09, 2025
-
How Are Compounds And Mixtures The Same
Apr 09, 2025
-
Distance Between Earth And Sun In Metres
Apr 09, 2025
Related Post
Thank you for visiting our website which covers about Why Is Sigma Bond Stronger Than Pi Bond . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.