Why Is Ethanol Soluble In Water
Juapaving
Mar 23, 2025 · 6 min read
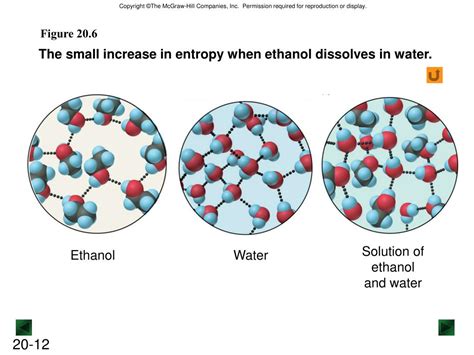
Table of Contents
Why is Ethanol Soluble in Water? A Deep Dive into Polarity and Hydrogen Bonding
Ethanol, a simple alcohol with the chemical formula CH₃CH₂OH, is remarkably soluble in water. This seemingly straightforward observation hides a fascinating interplay of intermolecular forces, specifically hydrogen bonding and polarity. Understanding why ethanol dissolves so readily in water requires a close look at the molecular structures and the interactions between them. This article will delve deep into the science behind ethanol's water solubility, exploring the concepts of polarity, hydrogen bonding, and the limitations of this solubility.
Understanding Polarity: The Key to Solubility
The principle governing the solubility of one substance in another is the famous adage, "like dissolves like." This means that polar substances tend to dissolve in polar solvents, while nonpolar substances dissolve in nonpolar solvents. To understand why ethanol dissolves in water, we must first grasp the concept of polarity.
Polarity arises from the unequal sharing of electrons in a covalent bond. Electronegativity, the ability of an atom to attract electrons in a bond, plays a crucial role. When atoms with significantly different electronegativities bond, the more electronegative atom attracts the shared electrons more strongly, creating a partial negative charge (δ-) on that atom and a partial positive charge (δ+) on the less electronegative atom. This unequal distribution of charge creates a dipole moment, making the molecule polar.
Water (H₂O) is a prime example of a polar molecule. Oxygen is significantly more electronegative than hydrogen, leading to a partial negative charge on the oxygen atom and partial positive charges on the hydrogen atoms. This creates a strong dipole moment, making water a highly polar solvent.
Ethanol also exhibits polarity. The oxygen atom in the hydroxyl group (-OH) is more electronegative than both carbon and hydrogen. This creates a partial negative charge on the oxygen and partial positive charges on the hydrogen of the hydroxyl group and the carbons. While the ethyl group (CH₃CH₂) is relatively nonpolar, the presence of the polar hydroxyl group dominates the overall polarity of the ethanol molecule.
The Power of Hydrogen Bonding: A Strong Intermolecular Force
While polarity is crucial for solubility, the strength of the interaction between ethanol and water molecules is further enhanced by hydrogen bonding. Hydrogen bonding is a special type of dipole-dipole interaction that occurs when a hydrogen atom bonded to a highly electronegative atom (like oxygen or nitrogen) is attracted to a lone pair of electrons on another electronegative atom in a different molecule.
In the ethanol-water system, hydrogen bonds form between the oxygen atom of one molecule and the hydrogen atom of the hydroxyl group (-OH) of another molecule. The oxygen atom in water can form hydrogen bonds with the hydrogen atom of the hydroxyl group in ethanol, and the oxygen atom in ethanol can form hydrogen bonds with the hydrogen atoms in water. These hydrogen bonds are relatively strong intermolecular forces, significantly contributing to the solubility of ethanol in water.
Visualizing the Interactions
Imagine water molecules, each with a slightly positive hydrogen and a slightly negative oxygen, interacting with ethanol molecules. The slightly negative oxygen of water is attracted to the slightly positive hydrogen of the hydroxyl group in ethanol, forming a hydrogen bond. Simultaneously, the slightly positive hydrogen of water is attracted to the slightly negative oxygen of the hydroxyl group in ethanol, forming another hydrogen bond. These multiple hydrogen bonds create a strong network of interactions between ethanol and water molecules, effectively "dissolving" the ethanol.
Factors Affecting Ethanol Solubility in Water
While ethanol is highly soluble in water, the extent of its solubility is not unlimited. Several factors influence how much ethanol can dissolve in water:
-
Temperature: Generally, solubility increases with temperature. Higher temperatures provide more kinetic energy to overcome intermolecular forces, allowing more ethanol molecules to disperse among water molecules.
-
Concentration: As the concentration of ethanol increases, the solubility may reach a limit. At saturation, no more ethanol can dissolve in the water. Beyond this point, any additional ethanol would remain as a separate phase.
-
Presence of other solutes: The presence of other dissolved substances can affect ethanol's solubility. These solutes may compete for hydrogen bonding sites or alter the overall polarity of the solution, influencing ethanol's ability to dissolve.
Comparing Ethanol Solubility to Other Alcohols
The solubility of alcohols in water generally decreases as the length of the hydrocarbon chain increases. Methanol (CH₃OH), the smallest alcohol, is completely miscible with water (meaning they mix in any proportion). Ethanol is also highly soluble. However, as we move to larger alcohols like propanol (CH₃CH₂CH₂OH) and butanol (CH₃CH₂CH₂CH₂OH), the solubility decreases. This is because the longer hydrocarbon chains become increasingly nonpolar, reducing the overall polarity of the molecule and weakening the interaction with water. The larger hydrophobic (water-repelling) hydrocarbon chain dominates the interaction with the water molecules. The hydrogen bonding from the hydroxyl group becomes less effective in overcoming the hydrophobic interactions.
Practical Applications of Ethanol's Water Solubility
The remarkable solubility of ethanol in water has many practical applications across various industries:
-
Beverage Industry: Ethanol's solubility is fundamental to the production of alcoholic beverages. Ethanol readily dissolves in water, forming the basis of various alcoholic drinks.
-
Pharmaceutical Industry: Ethanol is a common solvent in pharmaceutical preparations. Its ability to dissolve both polar and some nonpolar compounds makes it valuable in formulating medications.
-
Cosmetics and Personal Care: Ethanol is a solvent in many cosmetic and personal care products, helping to dissolve ingredients and improve the product's texture and stability.
-
Fuel Industry: Ethanol is used as a biofuel, often blended with gasoline. Its solubility in water is important to consider when transporting and handling ethanol fuel blends.
Limitations and Considerations
While ethanol is highly soluble in water, it's important to note that the solubility is not infinite. At a certain concentration, a saturated solution is formed. Also, remember that the solubility is heavily influenced by the presence of other substances in the solution. Therefore, understanding the specific conditions, like temperature and presence of other solutes, is crucial for accurately predicting ethanol’s solubility in a given system.
Conclusion
The solubility of ethanol in water is a consequence of the interplay between its polarity and its ability to form hydrogen bonds with water molecules. The polar hydroxyl group (-OH) interacts strongly with the polar water molecules, forming multiple hydrogen bonds that contribute significantly to the solubility. While the ethyl group is non-polar, the strong polarity of the hydroxyl group and the hydrogen bonding interactions outweigh this effect, allowing for the high solubility of ethanol in water. However, it is crucial to remember that the solubility is not infinite and factors like temperature and the presence of other substances influence the extent of solubility. Understanding this intricate relationship between molecular structure, intermolecular forces, and solubility is vital in various scientific and industrial applications.
Latest Posts
Latest Posts
-
Dissolving Sugar In Water Is Which Change
Mar 24, 2025
-
Define The Following Terms Alleles Genotype Phenotype Genome
Mar 24, 2025
-
What Parts Of Daltons Atomic Theory Are Wrong
Mar 24, 2025
-
Which Is A Component Of The Biosphere
Mar 24, 2025
-
What Is 11 3 As A Mixed Number
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Why Is Ethanol Soluble In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
