Why Is A Pi Bond Stronger Than Sigma
Juapaving
Mar 22, 2025 · 5 min read
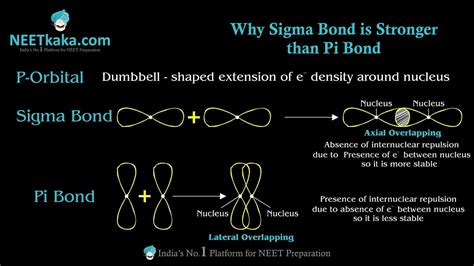
Table of Contents
Why is a Pi Bond Stronger Than a Sigma Bond? (A Deep Dive into Molecular Orbital Theory)
The statement "a pi bond is stronger than a sigma bond" is incorrect. In reality, a sigma (σ) bond is generally stronger than a pi (π) bond. This misconception often arises from a misunderstanding of how bond strength relates to bond order and the nature of sigma and pi bonds within a multiple bond. Let's delve into the detailed explanation, clarifying the relationship between bond strength, bond order, and the types of bonds involved.
Understanding Sigma (σ) and Pi (π) Bonds
Both sigma and pi bonds are covalent bonds formed by the overlap of atomic orbitals. However, they differ significantly in their geometry and electron density distribution.
Sigma (σ) Bonds
- Formation: A sigma bond is formed by the head-on overlap of atomic orbitals. This overlap is direct and leads to high electron density concentrated along the internuclear axis connecting the two bonded atoms. This means the electron cloud is directly between the two nuclei.
- Strength: Due to this direct and extensive overlap, sigma bonds are stronger and shorter than pi bonds. The electrons are held more tightly between the nuclei.
- Rotation: Sigma bonds allow free rotation around the bond axis. The electron density distribution is symmetrical, thus rotation doesn't affect the overlap significantly.
Pi (π) Bonds
- Formation: A pi bond is formed by the sideways or lateral overlap of atomic orbitals, typically p orbitals. This overlap is less effective than the head-on overlap of a sigma bond. The electron density is concentrated above and below the internuclear axis, but not directly between the nuclei.
- Strength: Because the overlap is less extensive, pi bonds are weaker and longer than sigma bonds. The electrons are less tightly held.
- Rotation: Pi bonds restrict rotation around the bond axis. Rotating the molecule would break the sideways overlap, requiring significant energy.
Bond Order and Bond Strength
Bond order is a crucial factor in determining bond strength. It represents the number of chemical bonds between a pair of atoms. A higher bond order generally indicates a stronger bond.
- Single Bond: A single bond consists of only one sigma (σ) bond. (Bond order = 1)
- Double Bond: A double bond consists of one sigma (σ) bond and one pi (π) bond. (Bond order = 2)
- Triple Bond: A triple bond consists of one sigma (σ) bond and two pi (π) bonds. (Bond order = 3)
While a triple bond is stronger than a double bond, and a double bond is stronger than a single bond, this strength is primarily due to the presence of additional sigma and pi bonds, not because the pi bonds are inherently stronger than the sigma bonds. The sigma bond always constitutes the fundamental framework of the bond.
Why Sigma Bonds are Stronger: A Deeper Look
The superior strength of sigma bonds arises from several factors:
-
Greater Overlap: The head-on overlap of atomic orbitals in a sigma bond results in a larger region of electron density between the nuclei. This increased electron density leads to stronger electrostatic attraction between the positively charged nuclei and the negatively charged electrons, hence stronger bond strength.
-
More Effective Shielding: The electron cloud in a sigma bond is directly between the two nuclei, providing more effective shielding of the positive nuclear charges from each other. This reduces the repulsive forces between the nuclei, further stabilizing the bond.
-
Symmetrical Electron Density: The symmetrical nature of the electron cloud in sigma bonds contributes to its stability and strength. This symmetrical distribution ensures optimal electrostatic interactions between the nuclei and the electrons.
Delocalization and Resonance
Some molecules exhibit resonance structures, where electrons are delocalized over multiple atoms. This delocalization can lead to increased bond order and overall bond strength, but this still doesn't make the pi bond stronger than a sigma bond, it increases the total bond strength through the distribution of the electron cloud.
The enhanced stability of these molecules due to resonance isn't a property inherent to pi bonds, but a result of the electron delocalization that involves both sigma and pi electrons. The delocalized electrons contribute to the overall stability and strength of the molecule but do not change the relative strength of individual sigma and pi bonds.
Misconceptions and Clarifications
The misunderstanding about pi bond strength often stems from the fact that pi bonds contribute to the overall strength of multiple bonds (double and triple bonds). A double bond is stronger than a single bond, and a triple bond is stronger than a double bond. This increased strength, however, is a result of the addition of a pi bond to an existing sigma bond, not because the pi bond itself is stronger.
The pi bond's contribution to the overall bond strength stems from its participation in the overall electron density of the bond. While the pi bond is weaker, it contributes extra electron density, increasing the overall bond order and thus strength of the multiple bond.
Conclusion
In conclusion, a sigma bond is stronger than a pi bond. This difference arises from the nature of the atomic orbital overlap—head-on overlap for sigma bonds leads to greater electron density between nuclei and stronger bonding compared to the lateral overlap in pi bonds. While pi bonds contribute to the overall strength of multiple bonds, the increased strength comes from the combined effect of both sigma and pi bonds, not the inherent strength of the pi bond itself. Understanding the difference between bond strength and bond order is crucial to accurately interpret the properties of sigma and pi bonds and their contributions to molecular stability. The strength of a chemical bond is a complex interplay of several factors, and this must be considered when interpreting bond energy values.
Latest Posts
Latest Posts
-
Humans Belong To The Phylum And Class
Mar 22, 2025
-
Whats The Difference Between A Monitor And Tv
Mar 22, 2025
-
Derivative Of X With Respect To Y
Mar 22, 2025
-
Why Is The Melting Of Ice Not A Chemical Reaction
Mar 22, 2025
-
Is 3 4 Bigger Than 4 5
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Why Is A Pi Bond Stronger Than Sigma . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
