Why Activation Energy Is Not Affected By Temperature
Juapaving
Apr 06, 2025 · 5 min read
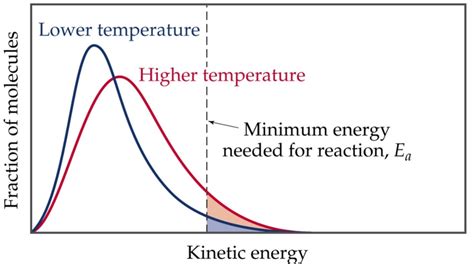
Table of Contents
Why Activation Energy Is Not Affected by Temperature
Activation energy is a fundamental concept in chemistry and physics, representing the minimum energy required for a reaction to occur. Understanding its relationship with temperature is crucial for comprehending reaction rates and chemical kinetics. A common misconception is that temperature affects activation energy. This article will delve deep into why this isn't the case, exploring the underlying principles and providing illustrative examples.
Understanding Activation Energy
Before we address the core question, let's establish a firm grasp on what activation energy actually is. Imagine a chemical reaction as a ball rolling over a hill. The height of the hill represents the activation energy (Ea). The ball, representing the reactants, needs to possess sufficient energy to climb the hill and reach the other side, representing the products. This energy is not recovered but is needed to initiate the process.
Key characteristics of activation energy:
- Independent of Temperature: This is the crux of our discussion, and we will elaborate on this point extensively later in the article. Activation energy remains constant for a given reaction regardless of temperature changes.
- Specific to Reaction: Each chemical reaction has its own unique activation energy value. Some reactions have low activation energies (easily overcome), while others have high activation energies (requiring significant energy input).
- Related to Reaction Rate: The magnitude of activation energy directly influences the reaction rate. Lower activation energy translates to faster reaction rates because more molecules possess the necessary energy to overcome the barrier.
- Determined Experimentally: Activation energy can be determined experimentally using methods like the Arrhenius equation, which relates the rate constant of a reaction to temperature and activation energy.
The Arrhenius Equation: A Deeper Dive
The Arrhenius equation provides a mathematical relationship between the rate constant (k), activation energy (Ea), temperature (T), and the pre-exponential factor (A):
k = A * exp(-Ea/RT)
Where:
- k is the rate constant
- A is the pre-exponential factor (frequency factor) – representing the frequency of collisions with the correct orientation.
- Ea is the activation energy
- R is the ideal gas constant
- T is the absolute temperature (in Kelvin)
This equation highlights the exponential relationship between the rate constant and temperature. Notice that Ea is a constant within the equation. While the rate constant (k) changes with temperature, it's the exponential term that is affected, not Ea itself. Changing the temperature alters the fraction of molecules possessing sufficient energy to overcome the activation energy barrier, thus influencing the reaction rate. However, the height of the barrier (Ea) remains unchanged.
Why Temperature Doesn't Affect Activation Energy
The misconception that temperature affects activation energy often arises from a misunderstanding of how temperature influences reaction rates. Higher temperatures lead to faster reaction rates because more molecules have the kinetic energy needed to surpass the activation energy barrier. However, this doesn't mean the activation energy barrier itself is changing. It's the number of molecules with sufficient energy that's changing, not the energy requirement itself.
Consider this analogy: Imagine a group of people trying to climb a mountain (the activation energy barrier). Some people are stronger and can climb faster, regardless of the weather. Others might struggle. If the weather gets warmer (higher temperature), more people might attempt the climb, increasing the overall number of successful ascents (faster reaction rate). But the height of the mountain (activation energy) remains unchanged. The temperature doesn't alter the inherent difficulty of the climb.
Microscopic Perspective: Collision Theory
The collision theory offers another perspective on why activation energy remains unaffected by temperature. For a reaction to occur, reactant molecules must collide with sufficient energy and proper orientation. Temperature affects the frequency and energy of collisions. Higher temperatures lead to more frequent and more energetic collisions. However, the minimum energy required for a successful reaction (activation energy) remains the same. More molecules simply possess this minimum energy at higher temperatures, increasing the chances of successful collisions and, consequently, a faster reaction rate.
Catalyst's Role: Lowering Activation Energy
It's crucial to differentiate between the effect of temperature and the effect of catalysts. While temperature doesn't affect activation energy, catalysts do. Catalysts provide an alternative reaction pathway with a lower activation energy, thus accelerating the reaction rate without changing the temperature. This is because catalysts provide a different reaction mechanism, effectively reducing the energy barrier the reactants need to overcome. This is a fundamental difference: temperature influences the rate of reaction, while a catalyst alters the mechanism of the reaction, thereby lowering the activation energy.
Experimental Evidence and the Arrhenius Plot
The Arrhenius equation can be linearized:
ln(k) = ln(A) – Ea/RT
This allows for the experimental determination of activation energy. By plotting ln(k) against 1/T (Arrhenius plot), a straight line is obtained with a slope of –Ea/R. The slope remains constant regardless of temperature variations, further confirming that activation energy is independent of temperature. The experiment consistently shows that the activation energy is inherent to the reaction itself and doesn't change with temperature fluctuations.
Practical Implications and Applications
Understanding the temperature-independence of activation energy has numerous practical applications across various fields:
- Chemical Engineering: Designing chemical reactors and optimizing reaction conditions relies heavily on the accurate estimation of activation energy. This knowledge allows engineers to control and predict reaction rates efficiently, regardless of temperature fluctuations.
- Catalysis Research: Studying catalyst effectiveness involves determining the activation energy of reactions with and without catalysts. This enables the evaluation and optimization of catalyst performance.
- Materials Science: Understanding activation energies helps in predicting the stability and reactivity of materials at different temperatures, crucial for material selection and design.
Conclusion
The activation energy of a chemical reaction is a fundamental property inherent to that specific reaction. It represents the minimum energy required for reactants to transform into products, irrespective of temperature. While temperature significantly influences the reaction rate by altering the number of molecules possessing sufficient energy to overcome the activation energy barrier, it does not change the height of the barrier itself. This distinction is crucial for a comprehensive understanding of chemical kinetics and reaction dynamics. The Arrhenius equation and collision theory provide strong theoretical foundations to support this principle, while experimental observations further validate the temperature independence of activation energy. This knowledge forms the basis for numerous applications in various scientific and engineering disciplines.
Latest Posts
Latest Posts
-
5 Letter Word With A And S
Apr 07, 2025
-
What Is Dispersion Of Light In Physics
Apr 07, 2025
-
Which Nonmetal Is The Most Reactive
Apr 07, 2025
-
What Distinguishes A Substance From A Mixture
Apr 07, 2025
-
Is 3 8 Bigger Than 5 16
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about Why Activation Energy Is Not Affected By Temperature . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
