Which One Of The Following Is A Weak Acid
Juapaving
Mar 15, 2025 · 5 min read
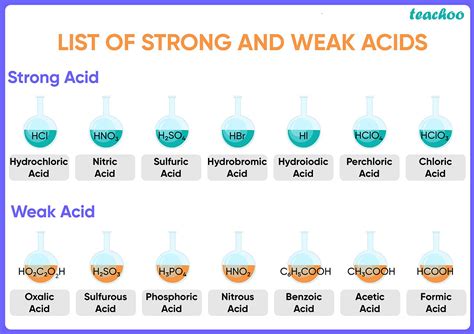
Table of Contents
Which One of the Following is a Weak Acid? Understanding Acid Strength
Determining whether an acid is weak or strong is crucial in chemistry, impacting everything from reaction rates to pH calculations. This article delves into the concept of weak acids, exploring their properties, distinguishing them from strong acids, and providing examples to solidify your understanding. We'll also explore the practical implications of understanding acid strength and provide strategies for identifying weak acids in various contexts.
What is a Weak Acid?
A weak acid is an acid that only partially dissociates (ionizes) in a solution. This means that only a small fraction of the acid molecules break down into ions (H⁺ and the conjugate base) when dissolved in water. Unlike strong acids, which dissociate almost completely, weak acids exist in equilibrium between the undissociated acid molecules and their ions. This equilibrium is represented by an equilibrium constant, Ka, which is a measure of the acid's strength.
Key Characteristics of Weak Acids:
- Partial Dissociation: A small percentage of the acid molecules donate a proton (H⁺) to water molecules.
- Equilibrium: The dissociation reaction is reversible, resulting in a dynamic equilibrium between the undissociated acid and its ions.
- Low Ka Value: Weak acids have a low acid dissociation constant (Ka), typically less than 1. The smaller the Ka value, the weaker the acid.
- Higher pH: For the same concentration, weak acids have a higher pH than strong acids because fewer H⁺ ions are present in the solution.
- Buffering Capacity: Weak acids, along with their conjugate bases, can act as buffers, resisting changes in pH upon the addition of small amounts of acid or base.
Strong Acids vs. Weak Acids: A Comparison
Understanding the differences between strong and weak acids is paramount. Here's a table summarizing the key distinctions:
| Feature | Strong Acid | Weak Acid |
|---|---|---|
| Dissociation | Complete dissociation in water | Partial dissociation in water |
| Ka Value | Very large (typically > 1) | Small (typically < 1) |
| pH | Low pH for a given concentration | Higher pH for a given concentration |
| Equilibrium | Essentially unidirectional | Reversible, exists in equilibrium |
| Examples | HCl (Hydrochloric acid), H₂SO₄ (Sulfuric acid), HNO₃ (Nitric acid) | CH₃COOH (Acetic acid), HF (Hydrofluoric acid), H₂CO₃ (Carbonic acid) |
Identifying Weak Acids: Practical Examples and Strategies
Identifying a weak acid often involves understanding its chemical structure and behavior in solution. Let's consider several common examples:
1. Organic Acids: The Carboxylic Acid Family
Many organic acids, especially those containing the carboxyl group (-COOH), are weak acids. This group is characterized by its ability to donate a proton (H⁺) to a base. Acetic acid (CH₃COOH), found in vinegar, is a prime example. Its relatively low Ka value indicates its weak acidic nature. Other examples include:
- Formic acid (HCOOH): The simplest carboxylic acid, found in ant stings.
- Lactic acid (CH₃CH(OH)COOH): Found in sour milk and muscles after strenuous exercise.
- Citric acid (C₆H₈O₇): Found in citrus fruits, a triprotic acid (can donate three protons).
2. Inorganic Weak Acids
While many inorganic acids are strong, some are weak. Hydrofluoric acid (HF) is a notable example. Despite fluorine being a highly electronegative element, the strong H-F bond makes it a relatively weak acid compared to other halogen acids like HCl, HBr, and HI.
Other inorganic weak acids include:
- Carbonic acid (H₂CO₃): Formed when carbon dioxide dissolves in water, playing a crucial role in blood buffering.
- Phosphoric acid (H₃PO₄): A triprotic acid used in fertilizers and food additives.
- Hydrogen sulfide (H₂S): A diprotic acid with a characteristic rotten egg smell.
3. Understanding the Factors Affecting Acid Strength
Several factors influence the strength of an acid:
- Electronegativity: The electronegativity of the atom bonded to the hydrogen atom influences the strength of the bond. More electronegative atoms make the bond more polar, facilitating proton donation.
- Bond Strength: Stronger bonds lead to weaker acids because more energy is required to break the bond and release the proton.
- Size of the Anion: Larger anions are more stable, making the acid stronger. The negative charge is more spread out, reducing the anion's attraction to the proton.
- Resonance: Delocalization of electrons through resonance stabilizes the conjugate base, increasing the acid's strength.
The Importance of Understanding Weak Acids
The knowledge of weak acids is critical across diverse fields:
- Medicine: Understanding the behavior of weak acids is vital in pharmacology for drug design and delivery. Many drugs are weak acids or bases, and their ionization affects their absorption and distribution in the body. Buffer systems based on weak acids maintain blood pH within a narrow physiological range.
- Environmental Science: Acid rain, caused by the release of sulfur dioxide and nitrogen oxides into the atmosphere, involves weak acids like sulfuric acid and nitric acid. Understanding their behavior is crucial for environmental remediation efforts.
- Industrial Chemistry: Many industrial processes utilize weak acids as catalysts or reactants. Their properties need to be considered for optimal reaction conditions.
- Analytical Chemistry: The determination of acid strength is essential in titration experiments and pH calculations.
Determining if an Acid is Weak: A Practical Approach
Given a list of acids, you can often determine which are weak by:
- Memorization: Familiarize yourself with common strong acids (HCl, HBr, HI, HNO₃, H₂SO₄, HClO₄). Any acid not on this list is likely weak.
- Ka Value: Refer to a table of Ka values. A low Ka value signifies a weak acid.
- Chemical Structure: Look for functional groups characteristic of weak acids, such as the carboxyl group (-COOH) in organic acids.
- Contextual Clues: The context in which the acid is used can provide clues. For instance, an acid used in a buffer solution is likely weak.
Conclusion: The Significance of Weak Acids in Various Disciplines
In conclusion, differentiating between strong and weak acids is a fundamental concept in chemistry with far-reaching implications. Understanding the properties of weak acids, their partial dissociation, their equilibrium constants (Ka), and the factors affecting their strength is crucial in various scientific disciplines. By mastering this concept, you gain the ability to better comprehend chemical reactions, predict the behavior of solutions, and apply this knowledge effectively in numerous contexts, from environmental science to medicine and industrial processes. Remember to utilize available resources like Ka tables and understand the chemical structures of potential acids to accurately identify whether an acid is weak or strong.
Latest Posts
Latest Posts
-
29 C Is What In Fahrenheit
Mar 15, 2025
-
How Many Cups Are In 6 Quarts
Mar 15, 2025
-
5 Letter Words Starting With Ap
Mar 15, 2025
-
Which One Of The Following Statements Is Correct
Mar 15, 2025
-
How Many Cubic Feet In A Quart
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Which One Of The Following Is A Weak Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
