Which Of The Following Is The Strongest Reducing Agent
Juapaving
Mar 22, 2025 · 5 min read
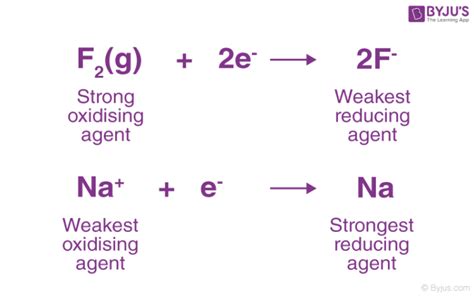
Table of Contents
Which of the Following is the Strongest Reducing Agent? A Deep Dive into Redox Chemistry
Determining the strongest reducing agent among a group of substances requires a fundamental understanding of redox chemistry, specifically reduction potentials and the electrochemical series. A reducing agent, by definition, is a substance that readily loses electrons, thereby causing the reduction of another substance. The stronger the reducing agent, the more readily it donates electrons. This article explores the factors influencing reducing power and provides a framework for comparing different reducing agents.
Understanding Reduction Potentials and the Electrochemical Series
The cornerstone of comparing reducing agents lies in their standard reduction potentials (E°). These values represent the tendency of a substance to gain electrons under standard conditions (298 K, 1 atm pressure, 1 M concentration). The electrochemical series, a table listing standard reduction potentials for various half-reactions, provides a crucial resource for this comparison. A more negative standard reduction potential indicates a stronger reducing agent. This is because a more negative potential signifies a greater tendency to lose electrons rather than gain them.
The logic is simple: A strong reducing agent will readily give away its electrons, undergoing oxidation itself while causing another substance to be reduced. The substance with the most negative E° value will be the most likely to undergo oxidation, making it the strongest reducing agent.
Factors Influencing Reducing Power
Several factors contribute to a substance's reducing power beyond just its standard reduction potential:
-
Electron Configuration: Elements with loosely held valence electrons tend to be stronger reducing agents. Alkaline earth metals (Group 2) and alkali metals (Group 1) are prime examples, readily losing their outermost electrons. The further away the valence electrons are from the nucleus, the easier it is for them to be donated.
-
Atomic Radius: Larger atoms generally have weaker attractions between the nucleus and their outermost electrons, making electron donation easier. This contributes to their higher reducing power.
-
Ionization Energy: Lower ionization energies indicate easier removal of electrons, correlating with stronger reducing power. Elements with low ionization energies easily lose electrons, thus acting as potent reducing agents.
-
Electronegativity: Electronegativity measures an atom's ability to attract electrons. Substances with low electronegativity are less likely to attract electrons and are thus more likely to donate them, exhibiting stronger reducing capabilities.
Comparing Reducing Agents: A Case Study
Let's consider a hypothetical scenario where we are asked to determine the strongest reducing agent from the following list: Lithium (Li), Sodium (Na), Potassium (K), and Magnesium (Mg).
Consulting the electrochemical series, we find the following standard reduction potentials (values may vary slightly depending on the source):
- Li⁺(aq) + e⁻ → Li(s) E° = -3.04 V
- Na⁺(aq) + e⁻ → Na(s) E° = -2.71 V
- K⁺(aq) + e⁻ → K(s) E° = -2.93 V
- Mg²⁺(aq) + 2e⁻ → Mg(s) E° = -2.37 V
Based solely on these standard reduction potentials, Lithium (Li) emerges as the strongest reducing agent because it has the most negative standard reduction potential (-3.04 V). This indicates its greater tendency to lose an electron and undergo oxidation compared to the other elements listed.
However, it's crucial to acknowledge that these values are under standard conditions. Deviations from standard conditions (temperature, pressure, concentration) can alter the relative reducing power. For instance, the concentration of the metal ions in solution significantly impacts the actual reduction potential. A higher concentration of metal ions will shift the equilibrium towards reduction, making the metal a weaker reducing agent. Conversely, a lower concentration will favor oxidation, enhancing the reducing power.
Beyond Standard Reduction Potentials: Practical Considerations
While standard reduction potentials offer a valuable starting point, other factors can influence the strength of a reducing agent in real-world applications:
-
Kinetic Factors: Even if a substance possesses a highly negative standard reduction potential, it might react slowly due to kinetic barriers. The activation energy required for the electron transfer might be substantial, hindering the reaction rate.
-
Solubility: The solubility of the reducing agent and its products impacts its effectiveness. A poorly soluble reducing agent might not be able to interact efficiently with the substance it's meant to reduce.
-
pH: The pH of the solution can significantly alter the reduction potential, affecting the reducing power of certain species.
-
Presence of other ions/complexes: The presence of other ions or complexing agents in the solution can also change the reduction potential and thereby influence the reducing power of the substance.
Extending the Analysis: More Complex Reducing Agents
The discussion so far has primarily focused on simple metal ions. However, many organic and inorganic compounds also exhibit significant reducing power. For instance, certain metal hydrides, such as sodium borohydride (NaBH₄) and lithium aluminum hydride (LiAlH₄), are powerful reducing agents in organic chemistry. Their reducing power stems from the hydride ion (H⁻), which readily donates electrons. Similarly, several organic molecules, such as ascorbic acid (vitamin C) and certain thiols, act as reducing agents due to their ability to donate electrons.
Applications of Strong Reducing Agents
Strong reducing agents have numerous applications in diverse fields:
-
Metallurgy: They are used in the extraction of metals from their ores. For example, carbon is used as a reducing agent in the extraction of iron from iron oxides.
-
Chemical Synthesis: They are crucial in organic and inorganic syntheses, facilitating various reduction reactions. Examples include the reduction of aldehydes and ketones to alcohols using reducing agents like sodium borohydride.
-
Batteries: Many batteries utilize redox reactions, with strong reducing agents acting as the anode (negative electrode), providing electrons that flow through the circuit.
-
Analytical Chemistry: Reducing agents are used in titrations and other analytical techniques to determine the concentration of oxidizing agents.
-
Environmental Remediation: Certain reducing agents are employed in cleaning up environmental pollutants by reducing harmful substances to less toxic forms.
Conclusion: A Holistic Approach to Assessing Reducing Power
Determining the strongest reducing agent among a given set of substances requires a multi-faceted approach. While standard reduction potentials provide a crucial first step, one must consider other factors like kinetic limitations, solubility, pH, and the presence of other species to gain a complete understanding of their reducing power in a specific context. Simply relying on the electrochemical series alone can be misleading, particularly in non-standard conditions. A thorough evaluation accounting for all relevant factors is necessary for an accurate determination of the strongest reducing agent. This holistic perspective is essential for successful applications in various fields ranging from materials science to environmental chemistry.
Latest Posts
Latest Posts
-
How Big Is 6 Inches In Cm
May 09, 2025
-
How Many Feet In 85 Inches
May 09, 2025
-
Does The Diagonals Of A Parallelogram Bisect Each Other
May 09, 2025
-
Is 88 A Prime Or Composite Number
May 09, 2025
-
Which Of The Following Bonds Is The Weakest
May 09, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Is The Strongest Reducing Agent . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.