Which Of The Following Is The Strongest Bond
Juapaving
Mar 13, 2025 · 6 min read
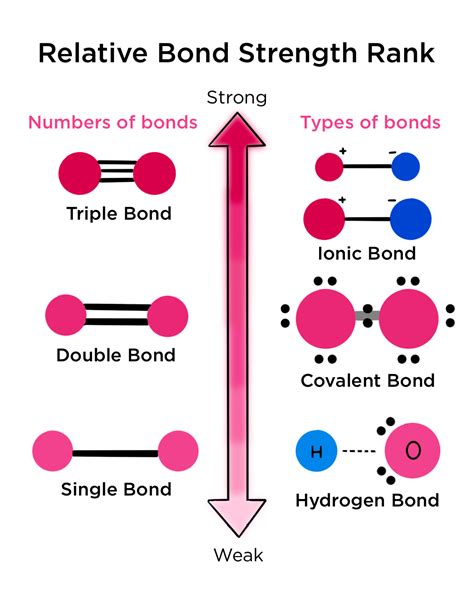
Table of Contents
Which of the Following is the Strongest Bond? A Deep Dive into Chemical Bonding
The question, "Which of the following is the strongest bond?" is deceptively simple. The answer hinges on understanding the nuances of various chemical bonds, the factors influencing their strength, and the context in which the comparison is made. This article will delve into the major types of chemical bonds – covalent, ionic, metallic, and hydrogen bonds – analyzing their strengths, weaknesses, and the properties they impart to the resulting compounds. We'll also explore factors like electronegativity, bond length, and bond order to provide a comprehensive understanding of bond strength.
Understanding Chemical Bonds: The Foundation of Matter
Chemical bonds are the forces that hold atoms together to form molecules and compounds. These forces arise from the electrostatic interactions between electrons and nuclei of atoms. The strength of a bond determines many properties of a substance, including its melting point, boiling point, solubility, and reactivity. Let's examine the key players:
1. Covalent Bonds: Sharing is Caring
Covalent bonds are formed when two atoms share one or more pairs of electrons. This sharing creates a stable electron configuration for both atoms, often resembling that of a noble gas. The strength of a covalent bond is directly related to the number of shared electron pairs (bond order) and the overlap of atomic orbitals.
- Single Bonds: A single covalent bond involves the sharing of one electron pair. These are generally weaker than multiple bonds.
- Double Bonds: Two electron pairs are shared, resulting in a stronger bond than a single bond.
- Triple Bonds: Three electron pairs are shared, forming the strongest type of covalent bond. The shorter bond length and increased electron density contribute to their strength.
Examples:
- Single Bond: H-H (hydrogen molecule)
- Double Bond: O=O (oxygen molecule)
- Triple Bond: N≡N (nitrogen molecule)
The strength of a covalent bond is also influenced by the electronegativity of the atoms involved. Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. If the electronegativity difference between the two atoms is significant, the bond will be polar covalent (e.g., H-Cl), possessing partial positive and negative charges. Purely nonpolar covalent bonds occur when atoms have identical or very similar electronegativity values (e.g., H-H). While the electronegativity difference influences polarity, it doesn't directly determine the overall strength of the covalent bond itself.
2. Ionic Bonds: Opposites Attract
Ionic bonds arise from the electrostatic attraction between oppositely charged ions. This occurs when one atom (typically a metal) readily loses electrons to become a positively charged cation, while another atom (usually a nonmetal) gains those electrons to become a negatively charged anion. The resulting electrostatic forces create the ionic bond.
The strength of an ionic bond depends on several factors:
- Charge of Ions: Higher charges on the ions lead to stronger attraction. For instance, a bond between Al³⁺ and O²⁻ will be stronger than a bond between Na⁺ and Cl⁻.
- Distance between Ions: Shorter distances between ions result in stronger bonds. Smaller ions generally form stronger ionic bonds.
- Lattice Energy: This refers to the energy released when gaseous ions combine to form a solid ionic lattice. Higher lattice energy indicates a stronger ionic bond.
Examples:
- NaCl (sodium chloride)
- MgO (magnesium oxide)
- CaF₂ (calcium fluoride)
Ionic bonds are generally stronger than single covalent bonds but typically weaker than double or triple covalent bonds.
3. Metallic Bonds: A Sea of Electrons
Metallic bonds occur in metals. The valence electrons of metal atoms are delocalized, meaning they are not associated with any particular atom but rather move freely throughout the metal lattice. This creates a "sea" of electrons that surrounds positively charged metal ions. The strong electrostatic attraction between the delocalized electrons and the metal ions is responsible for the metallic bond.
The strength of a metallic bond is influenced by:
- Number of Valence Electrons: More valence electrons contribute to a stronger metallic bond.
- Size of Metal Atoms: Smaller atoms generally form stronger metallic bonds.
Examples:
- Iron (Fe)
- Copper (Cu)
- Gold (Au)
Metallic bonds are responsible for many properties of metals, such as their malleability, ductility, and high electrical conductivity. The strength varies significantly across different metals.
4. Hydrogen Bonds: Special Interactions
Hydrogen bonds are a special type of dipole-dipole interaction that occurs when a hydrogen atom covalently bonded to a highly electronegative atom (such as oxygen, nitrogen, or fluorine) is attracted to another electronegative atom in a different molecule. It is not a true chemical bond in the same sense as covalent or ionic bonds, but it's a significant intermolecular force.
Hydrogen bonds are weaker than covalent and ionic bonds but play a crucial role in the structure and properties of many biological molecules, including water, proteins, and DNA.
Examples:
- Water (H₂O) molecules forming hydrogen bonds with each other.
- Hydrogen bonds between the bases in DNA.
Comparing Bond Strengths: The Verdict
Determining the "strongest" bond depends heavily on the specific context. Direct comparison across bond types is difficult, as the relative strength varies significantly. Generally, however, the following hierarchy holds true:
- Triple Covalent Bonds: These are typically the strongest bonds due to the high electron density and strong overlap of atomic orbitals.
- Double Covalent Bonds: Stronger than single covalent bonds, but weaker than triple covalent bonds.
- Ionic Bonds: Their strength depends on factors like charge and size of the ions but generally fall somewhere between single and double covalent bonds in terms of strength.
- Single Covalent Bonds: Weaker than double and triple covalent bonds and generally weaker than many ionic bonds.
- Metallic Bonds: The strength varies greatly among different metals, but are generally weaker than strong covalent or ionic bonds.
- Hydrogen Bonds: Significantly weaker than all the other bond types mentioned. However, their collective effect can be substantial, as seen in the properties of water and biological molecules.
Factors Influencing Bond Strength: A Deeper Dive
Beyond the basic type of bond, several other factors affect its strength:
- Bond Length: Shorter bond lengths generally correspond to stronger bonds. This is because the electrostatic attraction between the atoms is stronger at shorter distances.
- Bond Order: As mentioned earlier, higher bond order (more shared electron pairs) leads to stronger covalent bonds.
- Electronegativity: Although electronegativity affects bond polarity, it doesn't directly correlate with the overall strength of the bond. However, a very large difference in electronegativity could lead to a more ionic character, potentially weakening the covalent aspect of the bond.
- Steric Hindrance: Bulky groups surrounding the bonded atoms can hinder their close approach, weakening the bond.
- Resonance: In some molecules, electrons can be delocalized over multiple bonds, resulting in stronger, more stable bonds.
Conclusion: Context is Key
While triple covalent bonds are generally considered the strongest among the primary bond types, the actual strength of a bond is highly dependent on various factors. The context of the comparison is crucial. In some cases, a strong ionic bond might surpass a weaker covalent bond in strength. Ultimately, a comprehensive understanding of all factors influencing bond strength is necessary for accurate assessment. This article provides a solid foundation for comprehending the intricate world of chemical bonding and the relative strengths of various bonds. Further research into specific molecular systems will provide a deeper and more nuanced appreciation of this critical aspect of chemistry.
Latest Posts
Latest Posts
-
5 Letter Words Starting With A And Ending With Er
May 09, 2025
-
Difference Between A Hectare And An Acre
May 09, 2025
-
Most Widely Distributed Tissue Type In The Body
May 09, 2025
-
Difference Between Absorption Spectrum And Emission Spectrum
May 09, 2025
-
Does Evaporating A Liquid Increase Entropy
May 09, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Is The Strongest Bond . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.