Which Of The Following Is Non Metal
Juapaving
Mar 18, 2025 · 6 min read
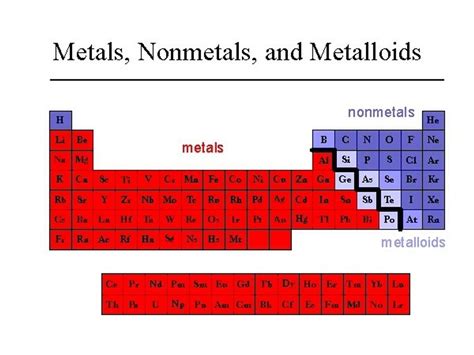
Table of Contents
Which of the Following is a Nonmetal? Understanding the Periodic Table
The periodic table is a cornerstone of chemistry, organizing elements based on their atomic structure and properties. One crucial classification within the table distinguishes between metals, nonmetals, and metalloids. While metals are generally known for their conductivity and malleability, nonmetals exhibit contrasting characteristics. Identifying a nonmetal often comes down to understanding these key differences. This article delves deep into the characteristics of nonmetals, providing clear examples and addressing common misconceptions.
Defining Nonmetals: Properties and Characteristics
Nonmetals are chemical elements that lack the typical characteristics of metals. This means they are generally poor conductors of heat and electricity, are brittle rather than malleable, and often exist as gases or brittle solids at room temperature. Unlike metals, they don't readily lose electrons to form positive ions. Instead, they tend to gain electrons, forming negative ions or sharing electrons to create covalent bonds.
Key Distinguishing Features of Nonmetals:
-
Poor Electrical Conductivity: Nonmetals are generally insulators, meaning they resist the flow of electric current. This contrasts sharply with metals, which are excellent conductors. The reason for this lies in the structure of their atoms and how tightly they hold onto their electrons.
-
Poor Thermal Conductivity: Similarly, nonmetals are poor conductors of heat. This property makes them useful in applications where insulation is required.
-
Brittle Nature: Unlike metals, which are typically malleable (can be hammered into sheets) and ductile (can be drawn into wires), nonmetals are brittle. They tend to shatter or crumble under stress.
-
Low Melting and Boiling Points: Many nonmetals have relatively low melting and boiling points compared to metals. This is because the forces holding their atoms together are weaker.
-
Dull Appearance: Nonmetals generally lack the shiny luster characteristic of metals. They often appear dull or have a variety of colors.
-
Formation of Negative Ions: Nonmetals tend to gain electrons to achieve a stable electron configuration, forming negatively charged ions (anions). This is a fundamental difference from metals, which tend to lose electrons to form positively charged ions (cations).
-
Covalent Bonding: Nonmetals often form covalent bonds with each other, sharing electrons rather than transferring them. This leads to the formation of molecules with diverse properties.
Examples of Nonmetals: A Comprehensive Overview
The periodic table neatly organizes nonmetals, primarily located on the right-hand side and in the upper right corner. Let's explore some prominent examples, highlighting their unique characteristics and applications:
Group 17: Halogens
The halogens (fluorine, chlorine, bromine, iodine, and astatine) are highly reactive nonmetals. Their reactivity stems from their tendency to gain one electron to achieve a stable electron configuration.
-
Fluorine (F): The most reactive element, fluorine is a pale yellow gas used in various applications, including the production of Teflon and other fluorocarbons. It's also crucial in dental health in the form of fluoride.
-
Chlorine (Cl): A greenish-yellow gas, chlorine is a powerful disinfectant used in water treatment and as a bleaching agent.
-
Bromine (Br): The only nonmetal that is liquid at room temperature, bromine is a reddish-brown liquid with a pungent odor. It finds applications in photography and flame retardants.
-
Iodine (I): A dark gray solid that sublimes (transitions directly from solid to gas), iodine is essential for thyroid function in humans and is used in antiseptic solutions.
-
Astatine (At): A rare and radioactive element, astatine's properties are less well-understood due to its instability.
Group 16: Chalcogens
The chalcogens (oxygen, sulfur, selenium, tellurium, and polonium) are another important group of nonmetals. Oxygen is essential for respiration, while sulfur plays a vital role in various biological processes and industrial applications.
-
Oxygen (O): A colorless, odorless gas essential for respiration in most living organisms. It is also a key component in combustion.
-
Sulfur (S): A yellow solid used in the production of sulfuric acid, a crucial industrial chemical. It's also found in some fertilizers and gunpowder.
-
Selenium (Se): A semiconductor with applications in electronics and photocopiers. It also plays a role as an antioxidant in the human body.
-
Tellurium (Te): A semiconductor used in specialized alloys and solar cells.
-
Polonium (Po): A rare and highly radioactive element with limited applications due to its toxicity and radioactivity.
Group 18: Noble Gases
The noble gases (helium, neon, argon, krypton, xenon, and radon) are unique nonmetals. They are exceptionally unreactive due to their complete electron shells, making them chemically inert.
-
Helium (He): Used in balloons, cryogenics, and MRI machines due to its low density and inertness.
-
Neon (Ne): Used in neon signs due to its characteristic orange-red glow when excited.
-
Argon (Ar): Used as an inert atmosphere in welding and other industrial processes.
-
Krypton (Kr): Used in some high-intensity lamps.
-
Xenon (Xe): Used in some specialized lighting applications and medical imaging.
-
Radon (Rn): A radioactive gas, radon is a health hazard due to its potential to cause lung cancer.
Other Notable Nonmetals:
-
Hydrogen (H): Although it occupies its own unique position on the periodic table, hydrogen is considered a nonmetal. It is the lightest element and forms many covalent compounds.
-
Carbon (C): A crucial element for life, carbon exists in various allotropes (different structural forms), including diamond, graphite, and fullerenes. Its ability to form long chains and rings is the basis for organic chemistry.
-
Nitrogen (N): A major component of Earth's atmosphere, nitrogen is crucial for plant growth and is used in the production of fertilizers and explosives.
-
Phosphorus (P): Exists in various allotropes, including white phosphorus (highly reactive) and red phosphorus (less reactive). It's vital for biological systems and is used in fertilizers and detergents.
Applications of Nonmetals: A Wide Range of Uses
Nonmetals play crucial roles in various industries and technologies. Their unique properties make them indispensable in a wide range of applications:
-
Electronics: Semiconductor nonmetals like silicon and germanium are fundamental to modern electronics.
-
Medicine: Halogens and other nonmetals are essential in various pharmaceuticals and medical devices.
-
Agriculture: Nitrogen and phosphorus are vital for fertilizers, ensuring crop production.
-
Energy: Hydrogen is being investigated as a potential clean energy source.
-
Manufacturing: Sulfur, carbon, and other nonmetals are crucial raw materials in various industrial processes.
-
Everyday Life: Numerous products we use daily, from plastics and fabrics to cleaning agents, incorporate nonmetals.
Distinguishing Between Metals, Nonmetals, and Metalloids
It's important to differentiate nonmetals from metals and metalloids, as their properties and behaviors differ significantly.
-
Metals: Conductors of electricity and heat, malleable, ductile, lustrous, tend to lose electrons. Examples: iron, copper, gold.
-
Nonmetals: Poor conductors, brittle, dull, tend to gain electrons. Examples: oxygen, chlorine, carbon.
-
Metalloids: Exhibit properties of both metals and nonmetals. Their conductivity can be influenced by factors such as temperature and pressure. Examples: silicon, germanium, arsenic. They often act as semiconductors.
Understanding these distinctions is vital for predicting chemical behavior and choosing appropriate materials for different applications.
Conclusion: The Importance of Understanding Nonmetals
Nonmetals, despite often being less prominently discussed than metals, are just as crucial in our world. Their unique properties and wide range of applications underscore their significance in various industries and technologies. From the oxygen we breathe to the silicon in our computers, nonmetals are integral to our daily lives. By understanding their characteristics and properties, we can better appreciate their contribution to scientific advancements and technological progress. This comprehensive overview hopefully clarifies the identity and importance of nonmetals within the broader context of the periodic table and their influence on our world. Further exploration of individual nonmetals and their unique roles in chemistry and various applications will enhance comprehension of this vital class of elements.
Latest Posts
Latest Posts
-
Torque And Moment Of Inertia Relationship
Mar 18, 2025
-
Is 1 3 Bigger Than 2 5
Mar 18, 2025
-
Tap Water Is Pure Substance Or Mixture
Mar 18, 2025
-
3 M Is How Many Cm
Mar 18, 2025
-
Two Angles Form A Linear Pair
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Is Non Metal . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
