Which Of The Following Is A Property Of Acid Solutions
Juapaving
Mar 21, 2025 · 7 min read
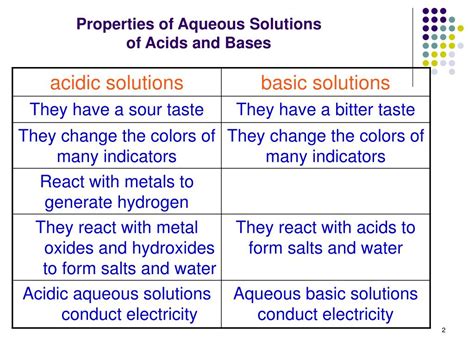
Table of Contents
Which of the Following is a Property of Acid Solutions?
Acids are ubiquitous in our lives, from the citric acid in oranges to the sulfuric acid used in car batteries. Understanding their properties is crucial in various fields, from chemistry and biology to environmental science and engineering. This article delves deep into the characteristics of acid solutions, exploring their defining properties and how they differ from other types of solutions. We'll examine the various tests used to identify acids, and discuss the implications of their properties in different contexts.
Defining Acids: More Than Just a Sour Taste
While the sour taste of many acids is a familiar characteristic, it's far from the only defining property. The scientific definition of an acid is much more precise. According to the Arrhenius definition, an acid is a substance that increases the concentration of hydrogen ions (H⁺) when dissolved in water. This definition, while useful, is limited as it only applies to aqueous solutions.
A broader and more comprehensive definition is provided by the Brønsted-Lowry theory, which defines an acid as a proton donor. This means an acid is any substance capable of donating a proton (H⁺) to another substance, the base. This theory expands the scope beyond aqueous solutions, encompassing reactions in non-aqueous solvents as well. Finally, the Lewis theory defines an acid as an electron-pair acceptor. This is the broadest definition, encompassing substances that don't even contain hydrogen but can still accept an electron pair from a base.
These definitions lay the foundation for understanding the various properties associated with acid solutions. Let's explore some key characteristics:
Key Properties of Acid Solutions
Several properties distinguish acid solutions from other types of solutions. These properties are often used to identify and characterize acids in the laboratory or in real-world applications.
1. Taste: Sourness (But Don't Taste Them!)
While this is a rudimentary test and should never be performed, many acids have a distinctly sour taste. This is a result of the hydrogen ions interacting with taste receptors on the tongue. However, it's crucial to emphasize the danger of tasting unknown substances. Many acids are corrosive and can cause severe damage to the mouth, throat, and esophagus. Never attempt to identify an acid by tasting it.
2. pH Value: Below 7
The pH scale is a logarithmic scale that measures the concentration of hydrogen ions (H⁺) in a solution. A pH of 7 indicates a neutral solution, while values below 7 indicate acidity, and values above 7 indicate alkalinity. Acid solutions have a pH less than 7, with stronger acids exhibiting lower pH values. The pH scale is a critical tool for characterizing the strength and concentration of acids.
3. Reaction with Metals: Hydrogen Gas Production
One of the most characteristic reactions of acids is their reaction with certain metals. Many acids react with active metals such as zinc, magnesium, and iron to produce hydrogen gas (H₂) and a metal salt. The general reaction can be represented as:
Acid + Metal → Salt + Hydrogen Gas
For example, the reaction between hydrochloric acid (HCl) and zinc (Zn) produces zinc chloride (ZnCl₂) and hydrogen gas:
2HCl(aq) + Zn(s) → ZnCl₂(aq) + H₂(g)
This reaction is often used as a test for the presence of acids. The evolution of hydrogen gas, often observable as bubbles, indicates the presence of an acid. However, the reactivity varies depending on the strength of the acid and the activity of the metal.
4. Reaction with Indicators: Color Change
Acid-base indicators are substances that change color depending on the pH of the solution. These indicators are often used to visually determine whether a solution is acidic or basic. Common indicators include litmus paper, phenolphthalein, and methyl orange.
- Litmus paper: Turns red in acidic solutions and blue in basic solutions.
- Phenolphthalein: Colorless in acidic solutions and pink in basic solutions.
- Methyl orange: Red in acidic solutions and yellow in basic solutions.
The color change is due to the interaction between the indicator molecules and the hydrogen ions in the solution, causing a change in the indicator's electronic structure and thus its color.
5. Conductivity of Electricity: Electrolytes
Acid solutions are good conductors of electricity. This is because acids dissociate into ions (H⁺ and the conjugate base anion) when dissolved in water. These ions are charge carriers, allowing the solution to conduct an electric current. The strength of the conductivity is related to the strength of the acid; stronger acids dissociate more completely, resulting in higher conductivity.
6. Reaction with Bases: Neutralization
Acids react with bases in a process called neutralization. This reaction results in the formation of water and a salt. The general equation for a neutralization reaction is:
Acid + Base → Salt + Water
For example, the neutralization reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) produces sodium chloride (NaCl) and water (H₂O):
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
Neutralization reactions are exothermic, meaning they release heat. The heat released can be used to determine the concentration of an acid or base using techniques like titration.
7. Corrosiveness: Damage to Materials
Many acids are corrosive, meaning they can damage or destroy other materials. The degree of corrosiveness varies depending on the strength and concentration of the acid. Strong acids, such as sulfuric acid and nitric acid, are highly corrosive and can cause severe burns to skin and damage to other materials. Weak acids, such as acetic acid (vinegar), are generally less corrosive but can still cause damage with prolonged exposure.
Differentiating Strong and Weak Acids
It's essential to differentiate between strong and weak acids. Strong acids completely dissociate into ions in water, meaning all the acid molecules break apart into their constituent ions. Examples include hydrochloric acid (HCl), sulfuric acid (H₂SO₄), and nitric acid (HNO₃). Weak acids, on the other hand, only partially dissociate in water, meaning only a small fraction of the acid molecules break apart into ions. Examples include acetic acid (CH₃COOH), carbonic acid (H₂CO₃), and hydrofluoric acid (HF).
The degree of dissociation determines the strength of the acid. Strong acids have a higher degree of dissociation and therefore a lower pH than weak acids at the same concentration.
Practical Applications of Acid Properties
The properties of acids are exploited in numerous applications across various fields:
1. Industrial Applications:
- Sulfuric acid: Used in the production of fertilizers, detergents, and batteries.
- Nitric acid: Used in the production of explosives and fertilizers.
- Hydrochloric acid: Used in the production of metals and cleaning agents.
- Phosphoric acid: Used in the production of fertilizers and food additives.
2. Biological Applications:
- Digestion: Hydrochloric acid in the stomach aids in digestion.
- Regulation of pH: Buffers in biological systems help maintain a constant pH.
3. Environmental Applications:
- Acid rain: Acidic pollutants in the atmosphere cause damage to ecosystems.
- Water treatment: Acids are used to adjust the pH of water.
4. Household Applications:
- Vinegar: (Acetic acid) Used as a cleaning agent and food ingredient.
- Citric acid: Used as a flavoring agent and preservative in foods and beverages.
Identifying Acids: A Summary of Tests
While tasting should never be attempted, several other tests can be used to identify acid solutions:
- pH measurement: Using a pH meter or indicator paper provides a quantitative measurement of acidity.
- Reaction with metals: Observing the evolution of hydrogen gas upon reaction with an active metal.
- Reaction with indicators: Observing a color change with acid-base indicators.
- Neutralization reaction: Titration with a known base can determine the concentration of the acid.
- Conductivity testing: High electrical conductivity suggests the presence of an acid.
Conclusion
Acid solutions possess a unique set of properties that distinguish them from other types of solutions. Understanding these properties – their sour taste (though never tested directly!), low pH, reaction with metals and bases, conductivity, and corrosiveness – is crucial for safe handling, practical applications, and a deeper understanding of chemistry. Remember to always prioritize safety when working with acids, following appropriate laboratory procedures and safety precautions. The information presented here provides a solid foundation for further exploration into the fascinating world of acids and their significance in various fields.
Latest Posts
Latest Posts
-
What Is The Difference Between Monarchy And Democracy
Mar 22, 2025
-
Difference Between Pl Sql And Sql
Mar 22, 2025
-
Tell Whether The Angles Are Adjacent Or Vertical
Mar 22, 2025
-
Surface Area Of A Cone Derivation
Mar 22, 2025
-
Where Is The Transitional Epithelium Located
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Is A Property Of Acid Solutions . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
