Which Of The Following Compounds Is Aromatic
Juapaving
Mar 10, 2025 · 6 min read
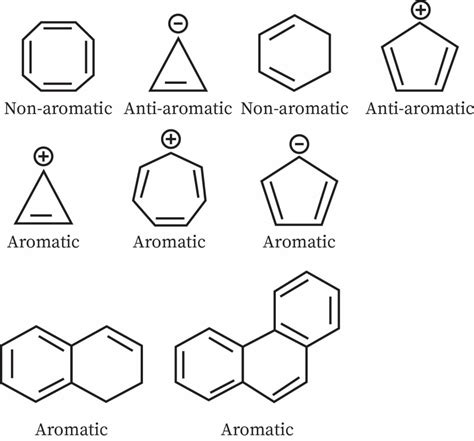
Table of Contents
Which of the Following Compounds is Aromatic? A Deep Dive into Aromaticity
Determining whether a compound is aromatic requires a thorough understanding of aromaticity rules. This article delves deep into the criteria for aromaticity, explaining each rule clearly and providing numerous examples to solidify your understanding. We'll explore various compounds, analyzing their structures to determine if they meet the requirements for aromaticity. By the end, you'll be able to confidently assess the aromaticity of a wide range of organic molecules.
The Four Rules of Aromaticity
A compound is considered aromatic if it fulfills all four of the following criteria:
-
Cyclic: The molecule must be a closed ring system. No open chains are aromatic.
-
Planar: All atoms in the ring must lie in the same plane. This allows for effective p-orbital overlap. Significant deviations from planarity disrupt aromaticity.
-
Conjugated: The molecule must have a continuous system of overlapping p-orbitals. This means that every atom in the ring must have a p-orbital that can participate in delocalized pi-electron bonding. Sp3 hybridized atoms disrupt conjugation.
-
Hückel's Rule: The molecule must contain (4n + 2) pi electrons, where 'n' is a non-negative integer (0, 1, 2, 3...). This rule dictates that only certain numbers of pi electrons contribute to aromaticity (2, 6, 10, 14, etc.).
Understanding Pi Electrons
Pi (π) electrons are crucial for aromaticity. They reside in p-orbitals that are perpendicular to the plane of the ring. These p-orbitals overlap, forming a delocalized pi electron cloud above and below the ring. This delocalization is the key factor that imparts aromatic stability.
Examples of Aromatic Compounds
Let's examine some classic examples of aromatic compounds and why they meet the criteria:
Benzene (C₆H₆)
Benzene is the quintessential aromatic compound. It's a six-membered ring with alternating single and double bonds.
- Cyclic: It's a ring.
- Planar: All carbons are sp² hybridized, ensuring planarity.
- Conjugated: The overlapping p-orbitals from each carbon atom form a continuous conjugated system.
- Hückel's Rule: It has 6 pi electrons (4n + 2, where n = 1).
Therefore, benzene is definitively aromatic.
Pyridine (C₅H₅N)
Pyridine is a six-membered ring containing five carbon atoms and one nitrogen atom.
- Cyclic: It's a ring.
- Planar: The nitrogen atom is sp² hybridized, maintaining planarity.
- Conjugated: The nitrogen atom's lone pair of electrons resides in a p-orbital, participating in the conjugated system.
- Hückel's Rule: It has 6 pi electrons (the nitrogen contributes one electron to the pi system).
Thus, pyridine is aromatic.
Pyrrole (C₄H₅N)
Pyrrole is a five-membered ring with four carbon atoms and one nitrogen atom.
- Cyclic: It's a ring.
- Planar: The nitrogen atom is sp² hybridized, maintaining planarity.
- Conjugated: The nitrogen's lone pair is part of the conjugated system.
- Hückel's Rule: It has 6 pi electrons (four from the carbons and two from the nitrogen lone pair).
Hence, pyrrole is aromatic.
Furan (C₄H₄O)
Furan is a five-membered ring containing four carbon atoms and one oxygen atom.
- Cyclic: It's a ring.
- Planar: The oxygen atom is sp² hybridized, maintaining planarity.
- Conjugated: One lone pair on the oxygen participates in the conjugated system.
- Hückel's Rule: It possesses 6 pi electrons (four from the carbons and two from one of the oxygen lone pairs).
Furan, therefore, is aromatic.
Examples of Non-Aromatic Compounds
Now let's look at some examples that fail to meet the aromaticity criteria:
Cyclooctatetraene (C₈H₈)
Cyclooctatetraene is an eight-membered ring with alternating single and double bonds. While it appears cyclic and conjugated, it's not planar. The molecule adopts a tub-shaped conformation to relieve strain, preventing effective p-orbital overlap. It also violates Hückel's rule, having 8 pi electrons (4n, where n = 2). Therefore, it's non-aromatic. It exhibits properties of a typical alkene.
Cyclobutadiene (C₄H₄)
Cyclobutadiene is a four-membered ring with alternating single and double bonds. It’s planar and conjugated. However, it only has 4 pi electrons, violating Hückel's rule (4n, where n = 1). This makes it anti-aromatic, meaning it's less stable than expected for a conjugated system. Anti-aromaticity is highly destabilizing.
1,3-Cyclopentadiene
This five-membered ring has two double bonds and one sp³ hybridized carbon. The presence of the sp³ hybridized carbon disrupts the conjugation, making it non-aromatic. The molecule readily deprotonates to form the cyclopentadienyl anion, which is aromatic (6 pi electrons).
Aromatic vs. Anti-aromatic vs. Non-Aromatic: A Comparison
| Feature | Aromatic | Anti-Aromatic | Non-Aromatic |
|---|---|---|---|
| Cyclic | Yes | Yes | Yes/No |
| Planar | Yes | Yes | No |
| Conjugated | Yes | Yes | No |
| Pi Electrons | 4n + 2 | 4n | Variable |
| Stability | Highly stable | Highly unstable | Moderate stability |
Determining Aromaticity: A Step-by-Step Approach
To determine if a compound is aromatic, follow these steps:
- Identify the ring: Is the molecule a cyclic structure?
- Assess planarity: Are all atoms in the ring sp² hybridized (or sp in some cases)? Is the molecule planar or does it have significant deviations from planarity?
- Check for conjugation: Is there a continuous system of overlapping p-orbitals?
- Count pi electrons: Apply Hückel's rule (4n + 2). If the number of pi electrons matches this rule, it's likely aromatic. If it's 4n, it's likely anti-aromatic. If it doesn't meet either condition, it's non-aromatic.
Remember to carefully consider lone pairs of electrons on heteroatoms (atoms other than carbon) as they may or may not contribute to the pi electron count.
Advanced Considerations
The rules of aromaticity provide a fundamental framework, but exceptions and complexities can arise. Factors like steric hindrance and strain can affect planarity and therefore aromaticity. Furthermore, understanding the concept of annulenes (monocyclic conjugated hydrocarbons) and their unique properties contributes to a comprehensive understanding of aromaticity. Exploring heterocyclic aromatic compounds (containing atoms other than carbon in the ring) expands the scope of this crucial concept in organic chemistry.
Conclusion
Determining whether a compound is aromatic requires a systematic evaluation of its structure according to the four criteria: cyclic, planar, conjugated, and adherence to Hückel's rule. This article has explored various examples, highlighting both aromatic and non-aromatic compounds and explaining the rationale behind their classification. By mastering these concepts, you'll gain a powerful tool for understanding the structure, properties, and reactivity of a vast array of organic molecules. Remember that a deep understanding of orbital hybridization, electron delocalization, and molecular geometry is crucial for confidently classifying compounds based on their aromatic properties.
Latest Posts
Latest Posts
-
113 097 Rounded To The Nearest Hundredth
May 09, 2025
-
Which Shell Do Transition Metals Remove Electrons From First
May 09, 2025
-
Opposite Angles Of A Parallelogram Are Equal
May 09, 2025
-
100 Cm Is How Many Mm
May 09, 2025
-
What Is An Extensive Physical Property
May 09, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Compounds Is Aromatic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.