Which Of The Following Are Considered Pure Substances
Juapaving
Mar 19, 2025 · 5 min read
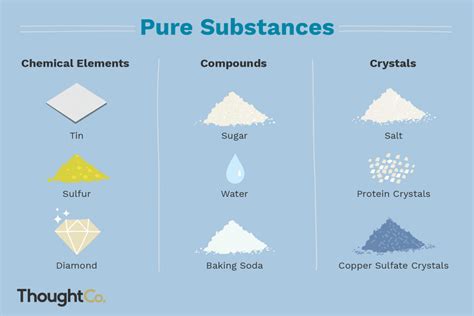
Table of Contents
Which of the Following are Considered Pure Substances? A Deep Dive into Matter
Understanding the difference between pure substances and mixtures is fundamental to chemistry. This article will delve deep into the definition of pure substances, exploring various types and providing numerous examples to solidify your comprehension. We'll clarify the distinction between elements, compounds, and mixtures, tackling common misconceptions along the way. By the end, you'll be equipped to confidently identify pure substances in any given scenario.
What is a Pure Substance?
A pure substance is a form of matter that has a constant composition and properties throughout the sample. This means that no matter where you take a sample from within the substance, its chemical makeup will be identical. It's crucial to understand that this definition hinges on the uniformity of the substance at a microscopic level. A pure substance cannot be separated into different components by physical methods like filtration, distillation, or decantation. Only chemical methods can break down a pure substance.
Two Main Types of Pure Substances:
Pure substances are broadly classified into two categories:
-
Elements: These are the fundamental building blocks of matter. Elements cannot be broken down into simpler substances by any chemical means. They are composed of only one type of atom. The periodic table organizes and displays all known elements. Examples include oxygen (O), gold (Au), hydrogen (H), and iron (Fe).
-
Compounds: Compounds are formed when two or more different elements chemically combine in a fixed ratio. This combination results in a completely new substance with properties distinct from its constituent elements. Compounds can only be broken down into their constituent elements through chemical processes, such as electrolysis or chemical reactions. Examples include water (H₂O), sodium chloride (NaCl – table salt), and carbon dioxide (CO₂).
Distinguishing Pure Substances from Mixtures
The key differentiator between pure substances and mixtures lies in their composition and properties. Mixtures, unlike pure substances, consist of two or more substances physically combined. These substances retain their individual properties within the mixture. Mixtures can be separated into their components by physical methods.
Types of Mixtures:
There are two main types of mixtures:
-
Homogeneous Mixtures: These mixtures have a uniform composition throughout. At a macroscopic level, they appear to be a single substance, even though they are composed of multiple components at a microscopic level. Examples include saltwater, air (a mixture of gases), and sugar dissolved in water.
-
Heterogeneous Mixtures: These mixtures have a non-uniform composition. Different components are visibly distinct and can be easily separated. Examples include sand and water, oil and water, and a salad.
Identifying Pure Substances: A Practical Approach
Let's apply these concepts to some everyday examples:
Examples of Pure Substances:
-
Distilled Water (H₂O): Distilled water is a pure substance because it's composed solely of water molecules. Tap water, on the other hand, is a mixture containing various dissolved minerals and impurities.
-
Pure Gold (Au): 24-karat gold is a pure element. Lower karat gold is an alloy (a mixture of gold and other metals).
-
Table Sugar (Sucrose, C₁₂H₂₂O₁₁): Table sugar is a pure compound, consisting of carbon, hydrogen, and oxygen atoms in a specific ratio.
-
Oxygen Gas (O₂): Pure oxygen gas is an element, made up only of oxygen atoms bonded together as diatomic molecules.
-
Diamond (C): A diamond is a pure form of carbon, specifically a crystalline allotrope.
Examples of Mixtures:
-
Seawater: Seawater is a homogeneous mixture containing water, salts, and other dissolved substances.
-
Air: Air is a homogeneous mixture of gases, primarily nitrogen and oxygen.
-
Milk: Milk is a heterogeneous mixture containing water, fats, proteins, and sugars.
-
Soil: Soil is a heterogeneous mixture containing various minerals, organic matter, and water.
-
Concrete: Concrete is a heterogeneous mixture of cement, sand, gravel, and water.
Common Misconceptions about Pure Substances
Several misconceptions frequently arise when discussing pure substances:
Misconception 1: Purity is Absolute
It's important to understand that "pure" in the context of chemistry doesn't mean absolutely free of any other substance. Instead, it implies a level of purity sufficient for the intended purpose. For example, while laboratory-grade chemicals aim for extremely high purity, traces of impurities might still exist.
Misconception 2: All Solids are Pure Substances
Many solids are mixtures. For example, rocks are heterogeneous mixtures of various minerals, and alloys (like steel) are mixtures of metals.
Misconception 3: Homogeneous Mixtures are Pure Substances
Homogeneous mixtures, while uniform in appearance, are not pure substances. They contain multiple substances physically combined.
Advanced Considerations: Allotropes and Isotopes
The concept of pure substances also involves understanding allotropes and isotopes:
Allotropes: Different Forms of the Same Element
Allotropes are different structural modifications of the same element. While they are made of the same element, their physical and chemical properties can differ significantly. For example, diamond and graphite are allotropes of carbon. Both are pure substances (carbon), but they have distinct properties due to the arrangement of their carbon atoms.
Isotopes: Atoms of the Same Element with Different Numbers of Neutrons
Isotopes are atoms of the same element that have the same number of protons but a different number of neutrons. While isotopes of an element have slightly different masses, they are still considered the same element and, therefore, a pure substance. For instance, carbon-12 and carbon-14 are isotopes of carbon; both are pure substances, but they have differing atomic masses.
Conclusion: Mastering the Identification of Pure Substances
Identifying pure substances requires a clear understanding of their definition and the differences between elements, compounds, and mixtures. By applying the principles outlined in this article, you'll be well-equipped to analyze various materials and confidently classify them as pure substances or mixtures. Remember to consider the uniformity of composition at a microscopic level, the possibility of separating components using physical methods, and the presence of multiple substances. Understanding these concepts is critical for progress in chemistry and related fields. The ability to distinguish between pure substances and mixtures underpins a solid foundation in scientific understanding. Continued learning and practice will further solidify this crucial knowledge.
Latest Posts
Latest Posts
-
Which Is More Important Heart Or Brain
Mar 19, 2025
-
What Is The Least Common Multiple Of 4 5 6
Mar 19, 2025
-
What Is The Lcm Of 2 3 And 4
Mar 19, 2025
-
How To Get A Percentage From A Ratio
Mar 19, 2025
-
How To Find Gcd Of 3 Numbers
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Are Considered Pure Substances . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
