Which Is The Correct Formula For Phosphorus Pentachloride
Juapaving
Mar 22, 2025 · 4 min read
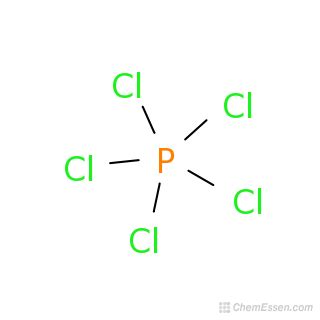
Table of Contents
Which is the Correct Formula for Phosphorus Pentachloride? Understanding Chemical Nomenclature and Structure
The correct formula for phosphorus pentachloride is PCl₅. This seemingly simple answer belies a fascinating exploration into chemical nomenclature, bonding, and the multifaceted nature of phosphorus chemistry. This article will delve deep into why PCl₅ is the correct formula, exploring the underlying principles and addressing common misconceptions. We will also touch upon its structure, reactivity, and importance in various chemical applications.
Understanding Chemical Nomenclature: The System Behind the Formula
Chemical nomenclature is the systematic way chemists name chemical compounds. It provides a universal language, ensuring that scientists worldwide understand each other. The name "phosphorus pentachloride" itself holds clues to its formula. Let's break it down:
-
Phosphorus (P): This refers to the element phosphorus, represented by its chemical symbol P. Phosphorus is a nonmetal in Group 15 of the periodic table, known for its ability to form multiple bonds.
-
Penta: This prefix signifies the presence of five atoms of the next element.
-
Chloride (Cl): This refers to the element chlorine, represented by its chemical symbol Cl. Chlorine, also a nonmetal, is highly reactive and readily forms bonds with other elements.
Therefore, combining these elements, "phosphorus pentachloride" translates directly to one phosphorus atom bonded to five chlorine atoms, leading to the formula PCl₅.
The Molecular Structure of Phosphorus Pentachloride: Beyond the Formula
While the formula PCl₅ accurately represents the stoichiometry (the ratio of atoms), it doesn't fully capture the compound's three-dimensional structure. Phosphorus pentachloride exhibits a trigonal bipyramidal geometry. This means the phosphorus atom sits at the center, surrounded by five chlorine atoms. Three chlorine atoms occupy equatorial positions (forming a triangular plane), and two chlorine atoms occupy axial positions (above and below the equatorial plane).
This geometry arises from the hybridization of phosphorus's atomic orbitals. Phosphorus utilizes its 3s, 3px, 3py, and 3pz orbitals, along with a vacant 3d orbital, to form five sp³d hybrid orbitals. Each of these hybrid orbitals overlaps with an orbital from a chlorine atom, forming five P-Cl sigma bonds. The slightly different bond lengths and energies between axial and equatorial P-Cl bonds are a consequence of this hybrid orbital arrangement.
Why not other formulas? Addressing common misconceptions
Some might wonder why other formulas, such as PCl₂ or PCl₁, are incorrect. This is because of phosphorus's valency. Phosphorus has five valence electrons, meaning it can form up to five covalent bonds. In PCl₅, phosphorus uses all five of its valence electrons to bond with five chlorine atoms, satisfying the octet rule (though phosphorus can exceed this rule) and achieving a stable configuration. Formulas like PCl₂ or PCl₁ would leave phosphorus with unpaired electrons, resulting in a highly reactive and unstable species.
The Reactivity of Phosphorus Pentachloride: A Versatile Compound
PCl₅ is a highly reactive compound, readily undergoing various chemical reactions. Its versatility stems from its ability to act as both a Lewis acid (electron-pair acceptor) and a chlorinating agent.
Lewis Acidity:
The phosphorus atom in PCl₅, despite having a complete octet, possesses a relatively low-lying vacant d orbital. This vacant d orbital can readily accept an electron pair from a Lewis base, forming an adduct. This Lewis acidity makes PCl₅ a useful reagent in various organic reactions.
Chlorinating Agent:
PCl₅ is a powerful chlorinating agent, meaning it can introduce chlorine atoms into other molecules. This property finds applications in organic synthesis for converting alcohols to alkyl chlorides, carboxylic acids to acyl chlorides, and many other transformations. The reaction typically involves the replacement of an -OH group with a -Cl group.
Applications of Phosphorus Pentachloride: From Synthesis to Industry
The diverse reactivity of phosphorus pentachloride makes it a crucial reagent in various fields:
-
Organic Synthesis: As mentioned above, its use in chlorination reactions is extensive, enabling the synthesis of a wide array of organic compounds.
-
Inorganic Chemistry: PCl₅ plays a role in the synthesis of other phosphorus-containing compounds and inorganic chlorides.
-
Industrial Applications: While less common now due to safety concerns, PCl₅ was previously used in certain industrial processes.
Safety Considerations: Handling Phosphorus Pentachloride
Phosphorus pentachloride is a corrosive and moisture-sensitive compound. Direct contact can cause severe burns to the skin and eyes. Inhalation of its vapors can also be harmful. Therefore, when working with PCl₅, proper safety precautions are essential:
-
Personal Protective Equipment (PPE): Always use appropriate PPE, including gloves, eye protection, and a lab coat.
-
Ventilation: Ensure adequate ventilation to minimize exposure to vapors.
-
Careful Handling: Handle PCl₅ with care, avoiding contact with skin and eyes.
Conclusion: The Definitive Formula and Beyond
The correct formula for phosphorus pentachloride is unequivocally PCl₅. Understanding this formula necessitates a grasp of chemical nomenclature, bonding theories, and the structural features of the molecule. The molecule's reactivity and various applications further highlight its importance in chemistry. While its direct industrial applications may be diminishing due to safer alternatives, its role as a versatile reagent in organic and inorganic synthesis remains crucial. Always remember to prioritize safety when handling this potent chemical. This in-depth understanding of PCl₅ extends beyond a simple formula; it represents a gateway to deeper exploration of chemical principles and their practical applications.
Latest Posts
Latest Posts
-
Ice Melting Physical Or Chemical Change
Mar 23, 2025
-
How Are Unicellular And Multicellular Alike
Mar 23, 2025
-
Which Statement About Viruses Is False
Mar 23, 2025
-
What Makes A Rule A Function
Mar 23, 2025
-
A Line That Intersects A Circle At Exactly Two Points
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Which Is The Correct Formula For Phosphorus Pentachloride . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
