Which Element Has The Highest Ionization Potential
Juapaving
Mar 06, 2025 · 5 min read
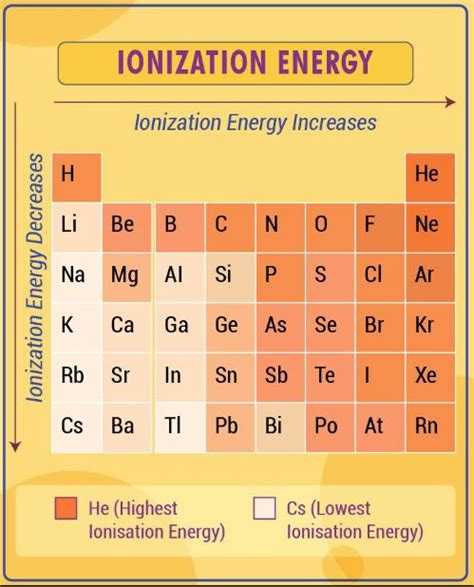
Table of Contents
Which Element Has the Highest Ionization Potential?
The quest to identify the element boasting the highest ionization potential is a fascinating journey into the heart of atomic structure and periodic trends. Understanding ionization potential, its relationship to electron configuration, and the factors influencing it are crucial to answering this question definitively. This exploration will delve deep into the intricacies of atomic physics, providing a comprehensive understanding not just of the answer, but the why behind it.
Understanding Ionization Potential (IP)
Ionization potential, also known as ionization energy, is the minimum amount of energy required to remove the most loosely bound electron from a neutral gaseous atom or ion. This process creates a positively charged ion (cation). It's a fundamental property of an element, reflecting the strength of the electrostatic attraction between the nucleus and its electrons. The higher the ionization potential, the more difficult it is to remove an electron, signifying a stronger hold on its electrons. This is measured in electron volts (eV) or kilojoules per mole (kJ/mol).
Successive Ionization Potentials
It's important to distinguish between the first ionization potential (IP1), the energy needed to remove the first electron, and subsequent ionization potentials (IP2, IP3, etc.). Each successive ionization potential is always higher than the previous one. This is because removing an electron leaves a positively charged ion, resulting in a stronger electrostatic attraction to the remaining electrons. The increasing difficulty in removing subsequent electrons makes higher ionization potentials progressively larger.
Factors Influencing Ionization Potential
Several factors contribute to an element's ionization potential:
1. Nuclear Charge (Z):
The number of protons in the nucleus (atomic number, Z) directly influences the positive charge. A higher nuclear charge exerts a stronger pull on the electrons, increasing the ionization potential. This is a dominant factor.
2. Atomic Radius:
The distance between the nucleus and the outermost electrons (atomic radius) plays a significant role. A smaller atomic radius leads to a stronger electrostatic attraction, resulting in a higher ionization potential. Electrons closer to the nucleus are more tightly bound.
3. Shielding Effect:
Inner electrons shield the outermost electrons from the full positive charge of the nucleus. This shielding effect reduces the effective nuclear charge experienced by the valence electrons. Elements with more inner electrons experience greater shielding, leading to a lower ionization potential.
4. Electron Configuration:
The arrangement of electrons in different energy levels and sublevels influences ionization potential. Elements with a stable electron configuration (e.g., noble gases with full valence shells) have exceptionally high ionization potentials because removing an electron disrupts this stability. Conversely, elements with a nearly full or half-full subshell often exhibit higher ionization potentials than expected due to extra stability associated with these configurations.
Periodic Trends in Ionization Potential
Ionization potential exhibits clear periodic trends across the periodic table:
Across a Period (Left to Right):
As you move from left to right across a period, the ionization potential generally increases. This is primarily due to the increasing nuclear charge. The additional protons increase the attraction to the electrons, despite the addition of electrons to the same shell. The shielding effect remains relatively constant within a period.
Down a Group (Top to Bottom):
As you move down a group, the ionization potential generally decreases. This is because the atomic radius increases significantly. The increased distance between the nucleus and the valence electrons weakens the electrostatic attraction, making it easier to remove an electron. The increased number of inner electrons also contributes to a stronger shielding effect.
Helium: The Contender for Highest Ionization Potential
While the general trends are clear, the element with the highest first ionization potential isn't immediately obvious due to subtle interactions between the factors discussed above. However, a strong contender emerges: Helium (He).
Helium possesses a very small atomic radius and a high nuclear charge relative to its number of electrons. Its electron configuration (1s²) is exceptionally stable, representing a completely filled electron shell. Removing an electron from helium requires overcoming this significant stability, resulting in a high first ionization potential.
Comparing Helium to Other Elements
While other elements may have higher second or subsequent ionization potentials (due to increased positive charge after electron removal), Helium stands out due to its exceptionally high first ionization potential. For example, hydrogen (H) has a lower first ionization potential because it only has one electron, which is less tightly bound than the two electrons in helium. Lithium (Li) also has a lower first ionization potential due to its larger atomic radius and the shielding effect of the inner electrons.
Why Helium? A Deeper Dive
Let's re-examine the factors influencing Helium's exceptionally high ionization potential:
- Strong Nuclear Charge (Z=2): Helium has a relatively high nuclear charge for its size, significantly influencing the attractive force on its electrons.
- Minimal Shielding: With only two electrons, the shielding effect is minimal. The outermost electrons experience a nearly full nuclear charge.
- Extremely Small Atomic Radius: The 1s orbital is exceptionally compact, bringing the electrons very close to the nucleus, maximizing the electrostatic attraction.
- Exceptional Stability: The 1s² configuration represents a complete electron shell, conferring significant stability to the helium atom. Removing an electron disrupts this stable arrangement, requiring a substantial amount of energy.
Addressing Potential Misconceptions
It's crucial to clarify some potential misunderstandings:
-
Highest Ionization Potential ≠ Highest Electronegativity: Although related, they are distinct properties. Electronegativity measures an atom's ability to attract electrons in a chemical bond, while ionization potential relates to the energy required to remove an electron from a neutral atom.
-
Successive Ionization Potentials: While Helium's first ionization potential is the highest among the elements, its subsequent ionization potentials will naturally be even higher, as discussed earlier. This doesn't change its position regarding the first ionization potential.
-
Relativistic Effects: At very high atomic numbers, relativistic effects start to become important and influence ionization potentials. However, these effects are negligible for lighter elements like Helium.
Conclusion: Helium Reigns Supreme
In conclusion, while the complexities of atomic structure and the interplay of different forces necessitate a nuanced understanding, Helium (He) stands out as the element with the highest first ionization potential. Its combination of small atomic radius, strong nuclear charge, minimal shielding, and the exceptional stability of its filled 1s² electron shell results in an exceptionally high energy requirement for electron removal. This makes helium incredibly unreactive and explains many of its unique physical and chemical properties. Understanding this phenomenon solidifies our comprehension of atomic behavior and the fundamental principles governing the periodic table.
Latest Posts
Latest Posts
-
Upsc Ese Photo And Signature Size
Mar 06, 2025
-
Whats The Square Root Of 12
Mar 06, 2025
-
Is 3 8 Bigger Than 1 2
Mar 06, 2025
-
Dna Can Be Found In What Two Organelles
Mar 06, 2025
-
What Are The Basic Building Blocks Of Matter
Mar 06, 2025
Related Post
Thank you for visiting our website which covers about Which Element Has The Highest Ionization Potential . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
