When Calcium Carbonate Is Added To Hydrochloric Acid
Juapaving
Mar 18, 2025 · 5 min read
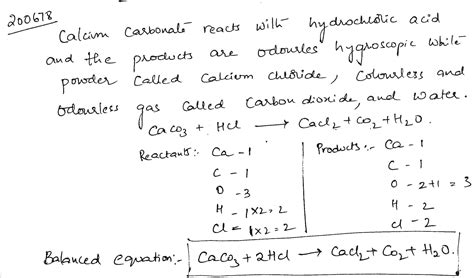
Table of Contents
When Calcium Carbonate is Added to Hydrochloric Acid: A Comprehensive Look at the Reaction
Calcium carbonate (CaCO₃), a common compound found in limestone, marble, and chalk, reacts vigorously with hydrochloric acid (HCl), a strong acid. This reaction is a classic example of an acid-base reaction, specifically a neutralization reaction, producing a salt, water, and carbon dioxide gas. Understanding this reaction is crucial in various fields, from chemistry education to industrial applications. This article delves deep into the intricacies of this reaction, covering its chemical equation, mechanism, applications, safety precautions, and variations.
The Chemical Reaction: A Detailed Explanation
The reaction between calcium carbonate and hydrochloric acid is an exothermic reaction, meaning it releases heat. The overall reaction can be summarized in the following balanced chemical equation:
CaCO₃(s) + 2HCl(aq) → CaCl₂(aq) + H₂O(l) + CO₂(g)
This equation illustrates that solid calcium carbonate reacts with aqueous hydrochloric acid to produce aqueous calcium chloride, liquid water, and gaseous carbon dioxide. Let's break down each component:
-
CaCO₃ (Calcium Carbonate): The reactant, a white, insoluble solid. It's a common component of many rocks and minerals.
-
HCl (Hydrochloric Acid): The reactant, a strong, corrosive acid. It's commonly used in laboratories and industrial processes.
-
CaCl₂ (Calcium Chloride): The product, a soluble salt. It's often used as a de-icer and in various industrial applications.
-
H₂O (Water): The product, a liquid formed as a result of the neutralization reaction.
-
CO₂ (Carbon Dioxide): The product, a gas released during the reaction. This is easily observable as effervescence (fizzing).
The Reaction Mechanism: A Step-by-Step Breakdown
The reaction occurs in several steps:
-
Protonation of Carbonate Ion: The hydrochloric acid dissociates completely in water into H⁺ (hydrogen ions) and Cl⁻ (chloride ions). The H⁺ ions then react with the carbonate ion (CO₃²⁻) present in the calcium carbonate. This is the key step in the neutralization process.
-
Formation of Carbonic Acid: The reaction between H⁺ and CO₃²⁻ forms carbonic acid (H₂CO₃):
CO₃²⁻(aq) + 2H⁺(aq) → H₂CO₃(aq)
-
Decomposition of Carbonic Acid: Carbonic acid is an unstable compound and readily decomposes into water and carbon dioxide:
H₂CO₃(aq) → H₂O(l) + CO₂(g)
-
Formation of Calcium Chloride: The calcium ions (Ca²⁺) from the calcium carbonate combine with the chloride ions (Cl⁻) from the hydrochloric acid to form calcium chloride:
Ca²⁺(aq) + 2Cl⁻(aq) → CaCl₂(aq)
These steps occur simultaneously, leading to the overall reaction as described earlier. The release of carbon dioxide gas is the most readily observable evidence of this reaction.
Factors Affecting the Reaction Rate
Several factors can influence the rate of this reaction:
-
Concentration of Hydrochloric Acid: A higher concentration of HCl leads to a faster reaction rate due to a greater number of H⁺ ions available to react with the carbonate ions.
-
Surface Area of Calcium Carbonate: Finely powdered calcium carbonate reacts faster than larger pieces due to the increased surface area exposed to the acid.
-
Temperature: Increasing the temperature increases the kinetic energy of the molecules, leading to more frequent and energetic collisions, and thus a faster reaction rate.
-
Presence of Catalysts: Certain catalysts can potentially speed up the reaction, though this isn't commonly employed in this specific reaction.
Applications of the Calcium Carbonate and Hydrochloric Acid Reaction
This seemingly simple reaction has numerous applications in various fields:
-
Digestion of Calcium Carbonate: In the human body, stomach acid (which contains HCl) helps to digest calcium carbonate present in food.
-
Analysis of Limestone and Minerals: The reaction is used in determining the calcium carbonate content in limestone, marble, and other minerals. The amount of carbon dioxide released can be precisely measured to calculate the calcium carbonate percentage.
-
Cleaning and Etching: The reaction's ability to dissolve calcium carbonate is utilized in cleaning applications, such as removing scale from surfaces. It's also used in etching processes for materials containing calcium carbonate.
-
Industrial Processes: The reaction finds use in various industrial processes, including the production of calcium chloride and the removal of impurities from industrial gases.
-
Chemical Education: This reaction is a cornerstone experiment in chemistry education, demonstrating acid-base reactions, gas evolution, and stoichiometry.
Safety Precautions
Both calcium carbonate and hydrochloric acid require careful handling. Specific safety measures include:
-
Eye Protection: Always wear safety goggles to protect your eyes from splashes.
-
Gloves: Wear appropriate gloves to prevent skin contact with the acid.
-
Ventilation: The reaction produces carbon dioxide gas, so adequate ventilation is necessary to prevent the buildup of CO₂ and ensure safe working conditions.
-
Acid Handling: Always add the acid to water slowly and cautiously, never the other way around, to prevent splashing and vigorous heat generation.
-
Disposal: Dispose of the reaction products according to local regulations. The waste should be neutralized before disposal.
Variations and Related Reactions
The fundamental reaction discussed above can be adapted or extended in various ways:
-
Using Other Acids: Other acids, like sulfuric acid (H₂SO₄) or nitric acid (HNO₃), can also react with calcium carbonate, although the products may differ.
-
Reactions with Other Carbonates: Other metal carbonates, like sodium carbonate (Na₂CO₃) or magnesium carbonate (MgCO₃), will also react with hydrochloric acid in similar fashion, producing the corresponding metal chloride, water, and carbon dioxide.
-
Stoichiometric Calculations: The reaction provides a great platform for practicing stoichiometric calculations, allowing students to calculate the theoretical yield of products based on the amount of reactants used.
Conclusion: A Versatile Chemical Reaction
The reaction between calcium carbonate and hydrochloric acid is a fundamental and versatile chemical reaction with significant implications across numerous disciplines. Understanding its mechanism, applications, and safety precautions is vital for anyone working with these chemicals. The reaction is a valuable tool in various analytical procedures, industrial processes, and educational settings. Its simplicity belies its importance, making it a continually relevant topic in chemistry. Further exploration of this reaction and its variations can lead to a deeper understanding of acid-base chemistry and its applications in the real world. The reaction serves as an excellent example of a classic chemical process with far-reaching practical uses. Its significance in various fields underscores the importance of studying and understanding fundamental chemical reactions.
Latest Posts
Latest Posts
-
Occupies Space Between Plasma Membrane And Nucleus
Mar 18, 2025
-
What Is The Roman Numeral For 22
Mar 18, 2025
-
Write 54 As A Product Of Prime Factors
Mar 18, 2025
-
Outer Layer Of A Plant Cell
Mar 18, 2025
-
Least Common Multiple Of 9 And 27
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about When Calcium Carbonate Is Added To Hydrochloric Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
