What's The Molar Mass Of Nh3
Juapaving
Mar 28, 2025 · 5 min read
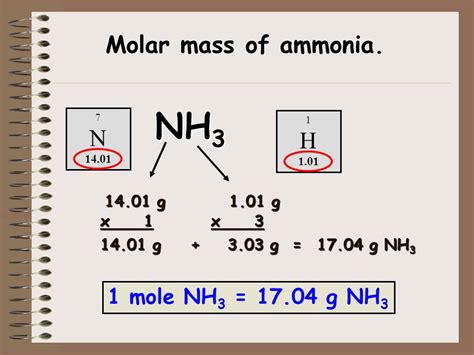
Table of Contents
What's the Molar Mass of NH₃? A Deep Dive into Ammonia's Properties
Ammonia (NH₃), a colorless gas with a pungent odor, plays a crucial role in various industrial processes and biological functions. Understanding its properties, particularly its molar mass, is essential for various applications, from fertilizer production to chemical analysis. This comprehensive guide delves into the calculation and significance of NH₃'s molar mass, exploring its implications in chemistry and beyond.
Understanding Molar Mass
Before calculating the molar mass of ammonia, let's establish a fundamental understanding of the concept. Molar mass is defined as the mass of one mole of a substance. A mole, in the context of chemistry, is a unit representing Avogadro's number (approximately 6.022 x 10²³ particles) of atoms, molecules, ions, or other specified entities. The molar mass is essentially the average atomic mass of a substance expressed in grams per mole (g/mol).
This value is crucial for numerous chemical calculations, enabling us to convert between mass, moles, and the number of particles. It's the bridge connecting the macroscopic world of grams and kilograms to the microscopic world of atoms and molecules.
Calculating the Molar Mass of NH₃
Ammonia (NH₃) is a simple molecule composed of one nitrogen atom (N) and three hydrogen atoms (H). To determine its molar mass, we need the atomic masses of nitrogen and hydrogen, readily available on the periodic table.
- Atomic mass of Nitrogen (N): Approximately 14.01 g/mol
- Atomic mass of Hydrogen (H): Approximately 1.01 g/mol
The molar mass of NH₃ is calculated by summing the atomic masses of its constituent atoms:
Molar Mass (NH₃) = Atomic Mass (N) + 3 * Atomic Mass (H)
Molar Mass (NH₃) = 14.01 g/mol + 3 * 1.01 g/mol
Molar Mass (NH₃) = 14.01 g/mol + 3.03 g/mol
Molar Mass (NH₃) = 17.04 g/mol
Therefore, the molar mass of ammonia (NH₃) is approximately 17.04 g/mol. This means that one mole of ammonia weighs approximately 17.04 grams.
Significance of Ammonia's Molar Mass
The molar mass of NH₃ is not merely a theoretical value; it holds significant practical implications across various fields:
1. Stoichiometric Calculations:
In chemical reactions involving ammonia, the molar mass is essential for performing stoichiometric calculations. Stoichiometry deals with the quantitative relationships between reactants and products in chemical reactions. Knowing the molar mass allows us to convert between the mass of ammonia and the number of moles, facilitating accurate predictions of reactant amounts and product yields.
For example, in the Haber-Bosch process (the industrial synthesis of ammonia), the molar mass of NH₃ is critical for determining the optimal ratios of nitrogen and hydrogen gases to maximize ammonia production.
2. Concentration Calculations:
Molarity, a common unit of concentration in chemistry, represents the number of moles of solute per liter of solution. The molar mass of NH₃ is crucial for preparing solutions of a specific molarity. If you need to prepare, say, a 1M solution of ammonia, you'll use the molar mass to determine the required mass of NH₃ to dissolve in a liter of solvent.
3. Gas Law Calculations:
The ideal gas law (PV = nRT) relates the pressure (P), volume (V), number of moles (n), temperature (T), and ideal gas constant (R) of a gas. The molar mass of NH₃ plays a vital role in calculating the number of moles (n) from the mass of ammonia, enabling us to apply the ideal gas law to solve problems related to ammonia's behavior under different conditions of temperature and pressure.
4. Applications in Industrial Processes:
The production and utilization of ammonia in numerous industries rely heavily on its molar mass. From the manufacturing of fertilizers to the synthesis of other chemicals, precise molar mass calculations ensure efficiency and quality control in these processes.
5. Analytical Chemistry:
In analytical chemistry, molar mass is fundamental to various techniques such as titration and gravimetric analysis. These techniques involve precise measurements of mass and volume, and accurate molar mass data is crucial for obtaining reliable analytical results. For example, in acid-base titrations involving ammonia, the molar mass helps determine the concentration of an unknown acid or base.
Beyond the Basics: Isotopes and Average Atomic Mass
The atomic masses used in our calculation (14.01 g/mol for Nitrogen and 1.01 g/mol for Hydrogen) are actually weighted averages of the isotopic masses of these elements. Nitrogen, for example, exists in two primary isotopes, ¹⁴N and ¹⁵N, with different natural abundances. The atomic mass of 14.01 g/mol represents the average mass, considering the contribution of each isotope weighted by its abundance.
This subtle detail highlights the fact that the molar mass of NH₃ is an average value, reflecting the natural isotopic distribution of nitrogen and hydrogen. In highly precise calculations, where isotopic composition might significantly impact the results, it is necessary to account for variations in isotopic abundance.
Practical Applications and Real-World Examples
Let's illustrate the practical use of NH₃'s molar mass with a couple of examples:
Example 1: Fertilizer Production
A fertilizer manufacturer needs to produce 1000 kg of ammonia. Using the molar mass of NH₃ (17.04 g/mol), we can calculate the number of moles required:
- Convert kg to g: 1000 kg * 1000 g/kg = 1,000,000 g
- Calculate moles: 1,000,000 g / 17.04 g/mol ≈ 58,678 moles of NH₃
This calculation enables the manufacturer to determine the required amounts of nitrogen and hydrogen for the Haber-Bosch process, ensuring efficient production.
Example 2: Determining Ammonia Concentration
A chemist needs to prepare 500 mL of a 0.5 M ammonia solution. Again, the molar mass plays a crucial role:
- Moles of NH₃ needed: 0.5 mol/L * 0.5 L = 0.25 moles
- Mass of NH₃ needed: 0.25 moles * 17.04 g/mol ≈ 4.26 g
The chemist would then weigh out 4.26 grams of ammonia and dissolve it in enough solvent to make a final volume of 500 mL.
Conclusion: The Importance of Precision
The molar mass of ammonia, precisely calculated at 17.04 g/mol, is a fundamental parameter in countless chemical applications. Understanding its calculation and significance is crucial for anyone working in chemistry, chemical engineering, or related fields. From stoichiometric calculations to industrial processes and analytical techniques, the accurate determination and application of ammonia's molar mass ensures precision and efficiency in various scientific and industrial endeavors. The examples presented in this comprehensive guide highlight the practical, real-world significance of this seemingly simple value. Remember that while the average molar mass is usually sufficient, in situations requiring high precision, consideration of isotopic variations may be necessary.
Latest Posts
Latest Posts
-
Which Of The Following Processes Returns Carbon To The Atmosphere
Mar 31, 2025
-
5 Letter Word That Starts With Vi
Mar 31, 2025
-
Can Acquired Characteristics Be Passed On The Next Generation
Mar 31, 2025
-
What Are The Three Body Parts Of A Mollusk
Mar 31, 2025
-
How Many Feet Is 45 Inches
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What's The Molar Mass Of Nh3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
