What's The Difference Between Exothermic And Endothermic
Juapaving
Mar 12, 2025 · 6 min read
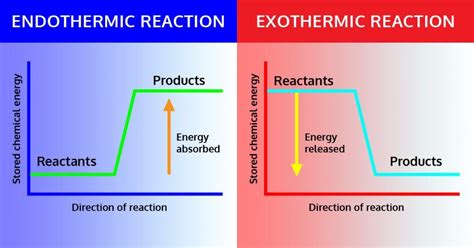
Table of Contents
What's the Difference Between Exothermic and Endothermic Reactions? A Deep Dive
Understanding the difference between exothermic and endothermic reactions is fundamental to grasping many chemical and physical processes. While seemingly simple at first glance, the nuances of these reactions extend far beyond just heat release or absorption. This comprehensive guide delves deep into the core concepts, exploring the mechanisms, examples, and practical applications of both exothermic and endothermic processes.
Defining Exothermic Reactions: Releasing Energy to the Surroundings
Exothermic reactions are characterized by the release of energy into their surroundings. This energy is typically manifested as heat, but it can also include light or sound. The word itself, "exothermic," originates from the Greek words "exo" (outside) and "thermos" (heat), aptly describing the outward flow of energy.
Key Characteristics of Exothermic Reactions:
-
Negative Change in Enthalpy (ΔH): This is the most crucial indicator. A negative ΔH signifies that the system (the reacting substances) has lost energy, and this energy is transferred to the surroundings. This means the products have less energy than the reactants.
-
Heat Release: The most readily observable characteristic is the generation of heat, often causing a temperature increase in the immediate environment. Think of a bonfire – the heat generated is a clear indication of an exothermic reaction.
-
Spontaneous Reactions (Often): While not always the case, many exothermic reactions occur spontaneously, meaning they don't require external energy input to initiate. However, the rate of reaction can be affected by factors like activation energy.
-
Examples of Exothermic Reactions:
-
Combustion: The burning of fuels like wood, propane, or gasoline is a classic example. The rapid oxidation of these materials releases significant heat and light.
-
Neutralization Reactions: The reaction between an acid and a base, forming salt and water, is usually exothermic. The heat released is often noticeable, especially when strong acids and bases are involved.
-
Respiration: Cellular respiration, the process by which living organisms convert glucose into energy, is also exothermic. This controlled release of energy sustains life processes.
-
Nuclear Reactions (Fission): The splitting of atomic nuclei, such as in nuclear power plants, is an extremely exothermic process, releasing enormous amounts of energy.
-
Defining Endothermic Reactions: Absorbing Energy from the Surroundings
In contrast to exothermic reactions, endothermic reactions absorb energy from their surroundings. This energy absorption often results in a decrease in the temperature of the immediate environment. The term "endothermic" derives from the Greek words "endo" (inside) and "thermos" (heat), signifying the inward flow of energy.
Key Characteristics of Endothermic Reactions:
-
Positive Change in Enthalpy (ΔH): A positive ΔH indicates that the system has gained energy from its surroundings. The products possess more energy than the reactants.
-
Heat Absorption: The surrounding environment loses heat as the reaction proceeds, leading to a temperature drop. Think of an ice pack – the melting of the ammonium nitrate absorbs heat from its surroundings, making it feel cold.
-
Non-Spontaneous Reactions (Often): Many endothermic reactions are not spontaneous and require a continuous supply of energy to proceed.
-
Examples of Endothermic Reactions:
-
Melting Ice: The phase transition from solid ice to liquid water requires the absorption of heat. The ice absorbs energy from its surroundings, resulting in a cooling effect.
-
Photosynthesis: Plants absorb sunlight to convert carbon dioxide and water into glucose and oxygen. The energy from sunlight is essential for this endothermic process.
-
Cooking an Egg: Cooking an egg involves denaturing proteins, a process that requires energy input in the form of heat.
-
Electrolysis: The decomposition of water into hydrogen and oxygen through the application of an electric current is another example. The electrical energy is absorbed by the system.
-
Dissolving Ammonium Nitrate: Dissolving ammonium nitrate in water is a classic example of an endothermic reaction. The solution becomes significantly colder as it absorbs heat from its surroundings.
-
Visualizing Energy Changes: Energy Diagrams
Energy diagrams provide a visual representation of the energy changes during exothermic and endothermic reactions. They typically plot potential energy against the reaction coordinate (progress of the reaction).
Exothermic Reaction Energy Diagram:
An exothermic reaction's energy diagram shows the reactants at a higher energy level than the products. The difference in energy is released as heat, represented by the downward slope of the curve. The activation energy (Ea), the energy barrier that must be overcome for the reaction to proceed, is also depicted.
Endothermic Reaction Energy Diagram:
An endothermic reaction's energy diagram depicts the reactants at a lower energy level than the products. The difference in energy represents the heat absorbed from the surroundings. The activation energy (Ea) is still present but the overall energy change is positive.
Activation Energy: The Energy Barrier
Both exothermic and endothermic reactions require an initial energy input known as activation energy (Ea). This is the minimum energy required to initiate the reaction by breaking existing bonds and forming new ones. Even though exothermic reactions release energy overall, they still need this initial "push" to get started.
Practical Applications of Exothermic and Endothermic Reactions
The principles of exothermic and endothermic reactions underpin numerous technologies and everyday processes:
Exothermic Reactions:
- Power Generation: Combustion in power plants provides electricity, while nuclear fission powers nuclear reactors.
- Heating and Cooking: Burning fuels for heating homes and cooking food relies on exothermic reactions.
- Industrial Processes: Many industrial processes, like cement production and metal smelting, utilize exothermic reactions.
Endothermic Reactions:
- Refrigeration: Endothermic processes, such as the evaporation of refrigerants, are used in refrigerators and air conditioners to cool spaces.
- Chemical Synthesis: Many industrial chemical syntheses involve endothermic reactions requiring energy input.
- Medical Applications: Some endothermic processes are used in medical applications, such as certain types of cryotherapy (using cold temperatures for therapeutic purposes).
Factors Affecting Reaction Rates
Several factors influence the rate at which both exothermic and endothermic reactions occur:
-
Temperature: Increasing the temperature generally speeds up both types of reactions by increasing the kinetic energy of molecules, leading to more frequent and energetic collisions.
-
Concentration: Higher concentrations of reactants typically increase the reaction rate by increasing the frequency of collisions.
-
Surface Area: For reactions involving solids, a larger surface area exposes more reactant molecules, leading to faster reaction rates.
-
Presence of Catalysts: Catalysts lower the activation energy, speeding up both exothermic and endothermic reactions without being consumed in the process.
Differentiating Exothermic and Endothermic: A Summary Table
| Feature | Exothermic Reaction | Endothermic Reaction |
|---|---|---|
| Energy Change | Releases energy to surroundings | Absorbs energy from surroundings |
| ΔH (Enthalpy) | Negative | Positive |
| Temperature | Increases in the surroundings | Decreases in the surroundings |
| Spontaneity | Often spontaneous | Often non-spontaneous |
| Examples | Combustion, Neutralization, Respiration | Melting ice, Photosynthesis, Electrolysis |
Conclusion: A Fundamental Understanding
The distinction between exothermic and endothermic reactions is crucial for understanding a wide array of chemical and physical phenomena. By comprehending the energy changes, characteristic features, and practical applications of these reactions, we gain valuable insights into the fundamental principles governing our world. From the energy we consume to the processes sustaining life itself, these reactions play a vital role, making their study an essential aspect of chemistry and beyond. This deep dive has provided a comprehensive understanding, enabling you to confidently analyze and appreciate the diverse world of exothermic and endothermic processes.
Latest Posts
Latest Posts
-
Opposite Angles Of A Parallelogram Are Equal
May 09, 2025
-
100 Cm Is How Many Mm
May 09, 2025
-
What Is An Extensive Physical Property
May 09, 2025
-
How To Find Perimeter Of A Pyramid
May 09, 2025
-
How Is A Square And A Rhombus Different
May 09, 2025
Related Post
Thank you for visiting our website which covers about What's The Difference Between Exothermic And Endothermic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.