What Types Of Bonds Hold Amino Acids Together
Juapaving
Mar 22, 2025 · 6 min read
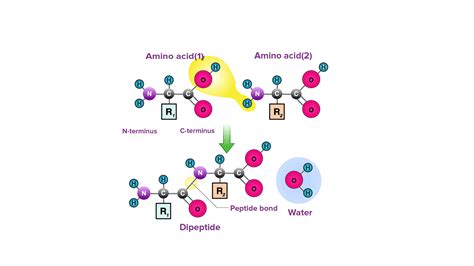
Table of Contents
What Types of Bonds Hold Amino Acids Together?
The structure and function of proteins are intricately linked to the types of bonds that hold amino acids together. Understanding these bonds is crucial to grasping the complexities of protein folding, stability, and biological activity. This comprehensive article will delve into the various types of bonds responsible for the primary, secondary, tertiary, and quaternary structures of proteins. We'll explore the characteristics of each bond, their strength, and their significance in determining protein properties.
The Primary Structure: The Peptide Bond
The primary structure of a protein refers to the linear sequence of amino acids linked together. This sequential arrangement is dictated by the genetic code and is fundamental to the protein's overall structure and function. The crucial bond responsible for this linear chain is the peptide bond, also known as an amide bond.
Characteristics of the Peptide Bond
The peptide bond is a covalent bond formed between the carboxyl group (-COOH) of one amino acid and the amino group (-NH2) of another amino acid. This reaction releases a molecule of water (H2O), making it a dehydration reaction. The bond itself is a C-N bond, but its characteristics are significantly different from a typical C-N single bond due to resonance.
-
Partial Double Bond Character: Due to resonance, the peptide bond exhibits partial double bond character. This means that the electrons are delocalized between the carbonyl oxygen and the amide nitrogen, resulting in a shorter and stronger bond than a typical single C-N bond. This partial double bond character restricts rotation around the peptide bond, influencing the protein's overall conformation.
-
Planarity: The peptide bond's partial double bond character leads to planarity. The six atoms involved in the peptide bond (carbonyl carbon, carbonyl oxygen, amide nitrogen, alpha-carbon of each amino acid, and the hydrogen atom on the amide nitrogen) lie in the same plane. This planarity is a critical factor influencing the protein's secondary structure.
-
Polarity: The peptide bond is polar due to the electronegativity difference between the oxygen and nitrogen atoms. This polarity contributes to the hydrogen bonding capabilities of the peptide backbone, playing a significant role in the secondary and tertiary structures.
Secondary Structure: Hydrogen Bonds and Beyond
The secondary structure of a protein refers to local, regular folding patterns of the polypeptide chain. The most common secondary structures are alpha-helices and beta-sheets. These structures are primarily stabilized by hydrogen bonds.
Hydrogen Bonds in Alpha-Helices and Beta-Sheets
Hydrogen bonds are weak non-covalent bonds that form between a partially positive hydrogen atom (attached to an electronegative atom like oxygen or nitrogen) and a partially negative electronegative atom. In proteins, these hydrogen bonds are crucial for stabilizing secondary structures.
-
Alpha-Helices: In an alpha-helix, the hydrogen bond forms between the carbonyl oxygen of one amino acid and the amide hydrogen of the amino acid four residues down the chain. These hydrogen bonds are parallel to the helical axis, creating a stable, rod-like structure.
-
Beta-Sheets: In beta-sheets, hydrogen bonds form between the carbonyl oxygen of one amino acid strand and the amide hydrogen of an amino acid in an adjacent strand. These hydrogen bonds are perpendicular to the polypeptide chain, creating a sheet-like structure. Beta-sheets can be parallel (strands run in the same direction) or antiparallel (strands run in opposite directions).
Other Bonds Influencing Secondary Structure
While hydrogen bonds are the dominant force stabilizing secondary structures, other interactions can contribute:
-
Hydrophobic Interactions: The clustering of nonpolar amino acid side chains within the protein core can influence the folding pattern and stability of secondary structures.
-
Van der Waals Forces: Weak, transient electrostatic interactions between atoms in close proximity can also play a minor role in stabilizing secondary structures.
Tertiary Structure: A Symphony of Interactions
The tertiary structure refers to the overall three-dimensional arrangement of a polypeptide chain, encompassing all its secondary structural elements. The tertiary structure is stabilized by a variety of interactions, including both covalent and non-covalent bonds.
Covalent Bonds in Tertiary Structure: Disulfide Bonds
Disulfide bonds are strong covalent bonds formed between the sulfur atoms of two cysteine residues. This bond is formed through an oxidation reaction, linking two polypeptide chains or different parts of the same chain. Disulfide bonds contribute significantly to the protein's stability and rigidity. They are particularly important in extracellular proteins that are exposed to harsh environments.
Non-Covalent Interactions in Tertiary Structure
Non-covalent interactions are crucial for shaping and stabilizing the tertiary structure. These interactions are individually weaker than covalent bonds but collectively exert a powerful influence.
-
Hydrogen Bonds: Hydrogen bonds between side chains of amino acids contribute to the precise folding of the protein.
-
Ionic Bonds (Salt Bridges): Ionic bonds form between oppositely charged amino acid side chains. For instance, a positively charged lysine residue can interact with a negatively charged aspartate residue.
-
Hydrophobic Interactions: Nonpolar amino acid side chains cluster together in the protein's hydrophobic core, away from the surrounding aqueous environment. This hydrophobic effect is a major driving force in protein folding.
-
Van der Waals Forces: These weak interactions contribute to the overall packing and stability of the protein's three-dimensional structure.
Quaternary Structure: The Assembly of Subunits
The quaternary structure describes the arrangement of multiple polypeptide chains (subunits) to form a functional protein complex. The same types of interactions that stabilize the tertiary structure are also essential for maintaining the quaternary structure.
Interactions Stabilizing Quaternary Structure
-
Hydrogen Bonds: Hydrogen bonds between the subunits contribute to their association.
-
Ionic Bonds: Salt bridges between charged amino acid side chains on different subunits help to maintain the complex.
-
Hydrophobic Interactions: The hydrophobic effect plays a significant role in the assembly and stability of multi-subunit proteins.
-
Disulfide Bonds: Disulfide bonds can link different polypeptide chains together, contributing to the stability of the quaternary structure.
Factors Affecting Bond Strength and Protein Stability
The strength of the bonds holding a protein together, and hence the stability of the protein, is influenced by various factors:
-
Temperature: High temperatures can disrupt weak non-covalent interactions, leading to protein denaturation.
-
pH: Changes in pH can alter the charges on amino acid side chains, disrupting ionic bonds and affecting protein stability.
-
Salt Concentration: High salt concentrations can shield charged groups, weakening ionic interactions.
-
Reducing Agents: Reducing agents can break disulfide bonds, leading to protein unfolding.
-
Chaperone Proteins: These proteins assist in the correct folding of proteins, preventing aggregation and misfolding.
Conclusion: A Complex Interplay of Forces
The structure and function of proteins are dictated by a complex interplay of covalent and non-covalent interactions. The peptide bond forms the backbone of the protein, while hydrogen bonds, ionic bonds, hydrophobic interactions, and disulfide bonds contribute to the secondary, tertiary, and quaternary structures. Understanding these bonds is critical to comprehending protein folding, stability, and function, and has profound implications in fields ranging from medicine to biotechnology. The delicate balance of these interactions allows proteins to perform their diverse roles in living organisms, making them essential molecules for life.
Latest Posts
Latest Posts
-
What Is The Molecular Mass Of Kno3
May 09, 2025
-
The Two Sides Of Dna Are Held Together By
May 09, 2025
-
Three Main Parts Of A Seed
May 09, 2025
-
Which Of The Following Is Strong Electrolyte
May 09, 2025
-
Why Do Skeletal Muscles Work In Pairs
May 09, 2025
Related Post
Thank you for visiting our website which covers about What Types Of Bonds Hold Amino Acids Together . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.