What Type Of Reaction Is Caco3 Cao Co2
Juapaving
Mar 22, 2025 · 6 min read
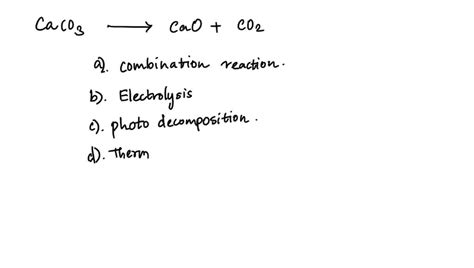
Table of Contents
What Type of Reaction is CaCO₃ → CaO + CO₂? A Deep Dive into Thermal Decomposition
The reaction CaCO₃ → CaO + CO₂ represents a fundamental chemical process with significant industrial and geological implications. Understanding its nature is crucial for grasping various concepts in chemistry, from reaction kinetics to thermodynamics. This comprehensive guide will delve into the specifics of this reaction, classifying it, exploring its mechanism, and discussing its applications and importance.
Classifying the Reaction: Thermal Decomposition
The reaction CaCO₃ → CaO + CO₂ is primarily classified as a thermal decomposition reaction. This classification arises from the fact that heat is the driving force behind the transformation of calcium carbonate (CaCO₃) into calcium oxide (CaO) and carbon dioxide (CO₂). Let's break down what this means:
-
Decomposition: Decomposition reactions involve a single compound breaking down into two or more simpler substances. In this case, the single reactant, calcium carbonate, decomposes into calcium oxide and carbon dioxide.
-
Thermal: The prefix "thermal" emphasizes the role of heat in initiating and sustaining the reaction. The reaction doesn't occur spontaneously at room temperature; it requires a significant input of thermal energy to overcome the activation energy barrier.
While it's primarily a thermal decomposition, we can also classify it further:
- Endothermic: The reaction absorbs heat from its surroundings. This is evident from the need for external heating to drive the process. The products (CaO and CO₂) possess higher energy than the reactant (CaCO₃).
Understanding the Mechanism: Breaking Bonds
At a molecular level, the thermal decomposition of calcium carbonate involves the breaking and forming of chemical bonds. The process is not instantaneous; it progresses through several steps, though the overall reaction is often represented as a single-step equation for simplicity. Let's examine the bond breaking involved:
-
Breaking the Carbonate Ion: The crucial step is the disruption of the carbonate ion (CO₃²⁻). This polyatomic ion is held together by strong covalent bonds between carbon and oxygen atoms. The application of heat provides the energy necessary to overcome these bonds, leading to the fragmentation of the ion.
-
Formation of Calcium Oxide and Carbon Dioxide: As the carbonate ion breaks apart, the carbon atom forms a double bond with one oxygen atom, creating carbon dioxide (CO₂). Simultaneously, the calcium cation (Ca²⁺) remains bound to the remaining oxygen atom, forming calcium oxide (CaO).
Activation Energy and Reaction Rate
The rate at which this thermal decomposition occurs is heavily influenced by the activation energy, the minimum energy required for the reaction to proceed. Factors such as temperature, surface area of the calcium carbonate, and the presence of catalysts significantly impact the reaction rate by affecting the activation energy. Higher temperatures provide more kinetic energy to the reactant molecules, increasing the likelihood of successful bond breaking and accelerating the reaction. Similarly, a larger surface area exposes more reactant molecules to heat, promoting faster decomposition.
Applications and Significance: Industrial Processes and Beyond
The thermal decomposition of calcium carbonate has extensive industrial and geological significance:
1. Lime Production: A Cornerstone of Many Industries
The primary industrial application is lime production. Calcium oxide (CaO), commonly known as quicklime or burnt lime, is a vital component in numerous industries:
-
Construction: Lime is a key ingredient in cement, mortar, and plaster, contributing to their setting and hardening properties. It's used in the production of concrete, a fundamental material in modern construction.
-
Steelmaking: Lime is used as a flux in steelmaking, helping to remove impurities from the molten iron.
-
Water Treatment: Lime is used to adjust the pH of water and to precipitate out impurities, improving water quality.
-
Agriculture: Lime is applied to acidic soils to neutralize their pH and improve their suitability for crop cultivation.
2. Geological Processes: Formation of Limestone and Marble
Geologically, this reaction is essential to understanding the formation and transformation of carbonate rocks. The process operates in reverse under specific conditions, leading to the formation of limestone and marble:
-
Limestone Formation: In aquatic environments, calcium ions (Ca²⁺) and carbonate ions (CO₃²⁻) precipitate out of solution, forming calcium carbonate (CaCO₃) deposits. Over long periods, these deposits accumulate, creating vast limestone formations.
-
Marble Formation: Limestone subjected to intense heat and pressure undergoes metamorphism, transforming into marble. This process is essentially the reverse of the thermal decomposition, though it's driven by geological forces rather than direct heating.
3. Other Applications: Chemical Synthesis and Analysis
Beyond these primary applications, the reaction also finds utility in various chemical processes:
-
CO₂ Production: The controlled decomposition of calcium carbonate serves as a convenient method for producing carbon dioxide in laboratories and certain industrial settings.
-
Chemical Analysis: The reaction's quantitative nature allows it to be used in gravimetric analyses to determine the calcium carbonate content in samples.
Factors Affecting Reaction Equilibrium: Le Chatelier's Principle
The thermal decomposition of calcium carbonate is a reversible reaction, meaning it can proceed in both the forward (decomposition) and reverse (formation of CaCO₃) directions. The position of equilibrium, which determines the relative amounts of reactants and products at equilibrium, is governed by factors such as temperature and pressure. Le Chatelier's principle elegantly describes this:
-
Temperature: Increasing the temperature shifts the equilibrium to the right, favoring the decomposition of CaCO₃ into CaO and CO₂. This is because the forward reaction is endothermic (heat-absorbing), and increasing the temperature adds more heat to the system, driving the reaction towards the products.
-
Pressure: Increasing the pressure shifts the equilibrium to the left, favoring the formation of CaCO₃. This is because the forward reaction produces more moles of gas (one mole of CO₂) than it consumes (zero moles of gas), increasing the total pressure. To alleviate the increased pressure, the equilibrium shifts towards the side with fewer moles of gas.
In summary, understanding Le Chatelier's principle is essential for controlling and predicting the outcome of this reversible reaction.
Advanced Concepts: Thermodynamics and Kinetics
The reaction's behavior can be further analyzed through the lens of thermodynamics and kinetics:
Thermodynamics: Gibbs Free Energy and Equilibrium Constant
Thermodynamic concepts like Gibbs free energy (ΔG) and the equilibrium constant (K) provide quantitative measures to describe the reaction's spontaneity and the extent of the decomposition at equilibrium. A negative ΔG indicates a spontaneous reaction under standard conditions, while the equilibrium constant determines the relative amounts of reactants and products at equilibrium. These values are temperature-dependent, explaining the shift in equilibrium with temperature changes.
Kinetics: Reaction Rate and Activation Energy
The rate of reaction is determined by kinetic factors such as the activation energy and the frequency of effective collisions between reactant molecules. As previously discussed, higher temperatures increase the reaction rate by providing sufficient kinetic energy to overcome the activation energy barrier.
Conclusion: A Reaction with Far-Reaching Impacts
The thermal decomposition of calcium carbonate, CaCO₃ → CaO + CO₂, is far from a simple chemical equation. It's a fundamental process with broad implications in industry, geology, and chemistry. Understanding its classification, mechanism, applications, and the thermodynamic and kinetic factors that govern its behavior provides invaluable insight into the intricacies of chemical reactions and their significance in the world around us. The reaction’s versatility and importance continue to drive research and innovation across multiple scientific and engineering disciplines. Its continuing study unravels deeper understandings of material behavior, reaction kinetics, and equilibrium principles – making it a truly fundamental concept in chemistry.
Latest Posts
Latest Posts
-
The Neutral Particle In The Nucleus Of An Atom
May 09, 2025
-
Label The Structures Of The Vertebrae
May 09, 2025
-
4 6 Rounded To The Nearest Tenth
May 09, 2025
-
If X Is A Multiple Of 18 And 60
May 09, 2025
-
Does Light Require A Medium To Travel
May 09, 2025
Related Post
Thank you for visiting our website which covers about What Type Of Reaction Is Caco3 Cao Co2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.