What Two Characteristics Do All Combustion Reactions Have In Common
Juapaving
Mar 19, 2025 · 7 min read
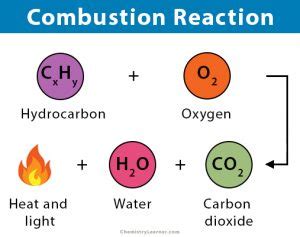
Table of Contents
What Two Characteristics Do All Combustion Reactions Have in Common?
Combustion, a fundamental chemical process, is responsible for much of the energy that powers our world, from the vehicles we drive to the electricity that lights our homes. Understanding its core characteristics is crucial in various fields, from engineering and environmental science to everyday life. While combustion reactions can appear diverse – from the controlled burn in a furnace to the uncontrolled blaze of a wildfire – they all share two fundamental characteristics: the presence of a fuel and an oxidant, and the release of heat and light (exothermic reaction). Let's delve deeper into each of these defining features.
1. The Presence of Fuel and Oxidant: The Necessary Ingredients
Combustion, at its heart, is a rapid oxidation reaction. This means it requires two key components: a fuel and an oxidant. The fuel is the substance that undergoes oxidation, providing the energy released during the process. The oxidant, typically oxygen (O₂), is the substance that accepts the electrons from the fuel, driving the reaction forward.
Understanding Fuel Sources: A Diverse Array
The term "fuel" encompasses a vast range of materials, both natural and synthetic. These fuels can be broadly categorized into:
-
Fossil Fuels: These are the most common fuels used today, derived from the remains of ancient plants and animals. They include:
- Coal: A solid fuel formed from compressed plant matter.
- Petroleum (crude oil): A liquid mixture of hydrocarbons extracted from underground reservoirs. It's refined into various products like gasoline, diesel, and kerosene.
- Natural Gas: Primarily methane (CH₄), a gaseous hydrocarbon found alongside petroleum.
-
Biomass: This refers to organic matter derived from recently living organisms. Examples include:
- Wood: A traditional fuel source, particularly in developing countries.
- Agricultural Residues: Crop stalks, straw, and other waste products from farming.
- Biofuels: Fuels produced from biomass, such as ethanol and biodiesel.
-
Other Fuels: This category includes a wider array of substances, including:
- Hydrogen (H₂): A clean-burning fuel that produces only water as a byproduct.
- Propane (C₃H₈): A gaseous hydrocarbon commonly used for heating and cooking.
- Acetylene (C₂H₂): A highly reactive gas used in welding and cutting.
The specific chemical composition of the fuel directly influences the products of combustion and the amount of energy released. For instance, the complete combustion of methane produces carbon dioxide (CO₂) and water (H₂O), while incomplete combustion can lead to the formation of carbon monoxide (CO), a highly toxic gas.
The Role of the Oxidant: More Than Just Oxygen
While oxygen is the most common oxidant in combustion reactions, other substances can also act as oxidizers under specific conditions. These include:
- Fluorine (F₂): A highly reactive halogen that readily oxidizes many substances.
- Chlorine (Cl₂): Another halogen that can support combustion, although less readily than fluorine.
- Nitric acid (HNO₃): A strong oxidizing agent often used in industrial processes.
The reactivity of the oxidant plays a crucial role in determining the rate and intensity of the combustion reaction. For example, a mixture of hydrogen and fluorine will combust much more vigorously than a mixture of hydrogen and oxygen.
2. Release of Heat and Light: An Exothermic Process
The second defining characteristic of all combustion reactions is their exothermic nature. This means that they release heat and light during the reaction. The energy released is a consequence of the breaking and forming of chemical bonds. The bonds in the fuel and oxidant are stronger than the bonds in the products, resulting in a net release of energy.
Understanding Enthalpy Change: Measuring the Energy Released
The amount of heat released during a combustion reaction is quantified by the enthalpy change (ΔH). This value is negative for exothermic reactions, indicating that energy is released. The magnitude of ΔH varies depending on the fuel and oxidant involved. For example, the combustion of methane has a significantly lower ΔH compared to the combustion of hydrogen.
The heat released during combustion has numerous practical applications:
- Power Generation: Combustion is the primary method for generating electricity in power plants, whether using fossil fuels, biomass, or other fuels.
- Heating and Cooking: Combustion provides heat for domestic and industrial applications, including heating homes and cooking food.
- Transportation: The combustion of gasoline and diesel fuel powers vehicles worldwide.
- Industrial Processes: Combustion plays a crucial role in various industrial processes, such as metal smelting and cement production.
The Visible Manifestation: Light and Flame
The release of light during combustion is often visible as a flame. The color and intensity of the flame vary depending on the temperature and the chemical composition of the fuel and products. For instance, a blue flame typically indicates complete combustion at a higher temperature, while a yellow or orange flame suggests incomplete combustion at a lower temperature.
Factors Affecting Combustion: Beyond the Basics
While the presence of fuel and oxidant and the release of heat and light are the defining characteristics of combustion, several other factors significantly influence the process:
- Temperature: A minimum ignition temperature is required to initiate combustion. This temperature varies depending on the fuel and oxidant involved.
- Concentration: The concentration of fuel and oxidant influences the rate and completeness of combustion. A stoichiometric mixture (the ideal ratio of fuel to oxidant) generally leads to the most efficient combustion.
- Pressure: Increasing the pressure generally increases the rate of combustion.
- Surface Area: A larger surface area of the fuel increases the contact between the fuel and oxidant, accelerating the reaction.
- Presence of Catalysts: Catalysts can accelerate combustion reactions by lowering the activation energy.
Applications of Combustion: Shaping Modern Society
Combustion plays a critical role in various aspects of modern life, powering much of our infrastructure and technology. However, its widespread use also presents significant challenges, particularly regarding environmental concerns.
Power Generation and Industrial Applications
Combustion is the cornerstone of electricity generation, utilizing fossil fuels and other energy sources in power plants to drive turbines and produce electricity. In industry, combustion is essential for processes like metal refining, cement manufacturing, and the production of numerous chemicals.
Transportation and Mobility
The internal combustion engine, reliant on the combustion of gasoline or diesel fuel, remains the dominant technology for powering vehicles. However, the environmental impact of these engines has spurred significant research into alternative fuels and propulsion systems.
Heating and Cooking: A Domestic Necessity
Combustion fuels our homes and provides the heat for cooking, through natural gas, propane, or other fuels. The ongoing development of energy-efficient appliances reduces the environmental footprint of domestic combustion.
Environmental Impacts and Mitigation Strategies
The widespread use of combustion poses environmental challenges, primarily due to the release of greenhouse gases, air pollutants, and other harmful substances.
Greenhouse Gas Emissions
The combustion of fossil fuels releases significant amounts of carbon dioxide (CO₂), a major greenhouse gas contributing to climate change. Mitigation strategies include transitioning to renewable energy sources, improving energy efficiency, and employing carbon capture and storage technologies.
Air Pollution
Combustion can produce various air pollutants, such as particulate matter, sulfur oxides, and nitrogen oxides. These pollutants negatively impact human health and the environment. Strategies to reduce air pollution include using cleaner fuels, installing emission control systems, and promoting sustainable transportation.
Conclusion: A Powerful Process with Environmental Responsibility
Combustion reactions are characterized by the presence of a fuel and an oxidant, along with the release of heat and light. This powerful process underpins many aspects of modern society, providing energy for electricity generation, transportation, heating, and various industrial processes. However, the environmental impact of combustion necessitates a shift towards cleaner and more sustainable energy sources and practices. Balancing the immense benefits of combustion with its environmental consequences remains a crucial challenge for the future. Ongoing research and technological advancements will be key to mitigating the negative effects while continuing to harness the power of combustion in a responsible and sustainable manner.
Latest Posts
Latest Posts
-
Describing Words That Start With D
Mar 20, 2025
-
What Gas Is Most Abundant In Earths Atmosphere
Mar 20, 2025
-
5 Letter Word Starting With T H O
Mar 20, 2025
-
The Elbow Is An Example Of What Type Of Joint
Mar 20, 2025
-
Why Does Active Transport Require Energy
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about What Two Characteristics Do All Combustion Reactions Have In Common . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
