What Metals Are Liquid At Room Temperature
Juapaving
Apr 01, 2025 · 7 min read
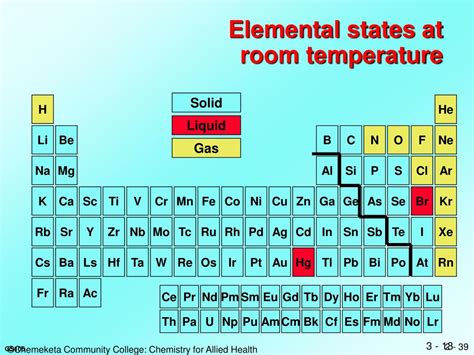
Table of Contents
- What Metals Are Liquid At Room Temperature
- Table of Contents
- What Metals Are Liquid at Room Temperature? A Deep Dive into Mercury and Beyond
- Mercury: The Only Truly Liquid Metal at Room Temperature
- Properties and Characteristics of Mercury
- Historical Uses and Current Applications of Mercury
- Beyond Mercury: Alloys and Other Considerations
- Amalgams: Liquid Metal Alloys
- Other Low-Melting-Point Metals and Alloys
- The Science Behind Liquid Metals at Room Temperature
- Future Possibilities and Research
- Conclusion: A Unique State of Matter
- Latest Posts
- Latest Posts
- Related Post
What Metals Are Liquid at Room Temperature? A Deep Dive into Mercury and Beyond
The question, "What metals are liquid at room temperature?" might seem simple at first glance. The immediate answer that springs to mind for most people is mercury. And while that's largely correct, the reality is more nuanced and fascinating than a simple one-word response. This article delves into the unique properties that allow some metals to remain liquid at temperatures comfortable for humans, explores the history and applications of these unusual materials, and touches on the scientific reasons behind their behavior. We'll also examine some related concepts and future possibilities.
Mercury: The Only Truly Liquid Metal at Room Temperature
Mercury (Hg), also known as quicksilver, is the most well-known and readily available liquid metal at standard room temperature (around 20°C or 68°F). Its liquid state at relatively low temperatures is due to its unique electronic structure and weak metallic bonding. Unlike most metals that solidify into a crystalline lattice structure at room temperature, mercury's electrons are more loosely held, leading to weaker interatomic forces and allowing it to remain fluid.
Properties and Characteristics of Mercury
Mercury possesses several distinctive properties:
- High Density: It is exceptionally dense, significantly heavier than most other common metals. This high density makes it useful in various applications, like barometers and manometers.
- High Surface Tension: Mercury exhibits a remarkably high surface tension, causing it to form nearly perfect spheres when dropped onto a non-reactive surface.
- Low Vapor Pressure: While liquid at room temperature, mercury still possesses a measurable vapor pressure, meaning it slowly evaporates. This vapor is highly toxic, hence the need for careful handling and safety precautions.
- Excellent Electrical Conductivity: Despite its liquid state, mercury remains a remarkably good conductor of electricity. This property is exploited in various electrical applications, although its toxicity restricts its widespread use.
- Toxicity: This is perhaps the most critical aspect of mercury. Both elemental mercury and its compounds are highly toxic, posing serious health risks through inhalation, ingestion, or skin absorption. The use of mercury is increasingly regulated due to its environmental and health hazards.
Historical Uses and Current Applications of Mercury
Mercury has a rich history of human use, spanning millennia. From ancient times, it was used in various applications:
- Alchemy: Central to alchemical practices, where it was believed to hold mystical properties.
- Medicine: Historically used in various medicinal preparations (now known to be extremely dangerous).
- Thermometers and Barometers: Its uniform thermal expansion made it ideal for creating accurate temperature measurement instruments. While largely replaced by safer alternatives, mercury thermometers are still found in some settings.
- Mining and Metallurgy: Used in the extraction of precious metals like gold.
- Electrical Switches and Relays: Its high conductivity made it suitable for certain electrical applications, though its toxicity has led to its phase-out.
Currently, mercury use is significantly restricted due to its toxicity. Its applications are gradually being replaced by safer alternatives in most fields. However, it still finds limited use in some specialized scientific instruments and industrial processes where its unique properties are essential.
Beyond Mercury: Alloys and Other Considerations
While mercury is the only pure metal liquid at room temperature, several metallic alloys exhibit this property. These are often created by combining mercury with other metals, forming amalgams. The properties of these amalgams can vary widely depending on the composition and proportions of the metals involved.
Amalgams: Liquid Metal Alloys
Amalgams are alloys of mercury with other metals. Some notable examples include:
- Dental Amalgam: Historically a common material for dental fillings, consisting primarily of mercury, silver, tin, and copper. Its use is declining due to concerns about mercury toxicity.
- Sodium Amalgam: Used in various chemical processes, particularly in the production of chlorine and caustic soda (sodium hydroxide).
- Gold Amalgam: Used in traditional gold mining techniques to aid in the extraction and purification of gold.
The liquid state of these amalgams is often attributed to the disruption of the crystalline structure of the other metals by the mercury atoms, reducing the interatomic forces and allowing the alloy to remain liquid at room temperature.
Other Low-Melting-Point Metals and Alloys
Beyond mercury and its amalgams, several other metals have relatively low melting points, bringing them close to being liquid at room temperature, though not quite reaching it under standard conditions:
- Cesium (Cs): With a melting point of 28.44°C (83.19°F), cesium is very close to being liquid at room temperature. It is a highly reactive alkali metal and requires careful handling.
- Gallium (Ga): Melting point of 29.76°C (85.57°F). Gallium's low melting point makes it an interesting material for various applications, including in semiconductors and in thermoelectric devices. It also has the unusual property of melting in the hand.
- Rubidium (Rb): Melting point of 39.31°C (102.76°F). Similar to cesium, rubidium is a highly reactive alkali metal.
These metals, while not strictly liquid at standard room temperature, highlight the range of melting points found in the metallic elements and underscore the factors contributing to a metal's phase at a given temperature.
The Science Behind Liquid Metals at Room Temperature
The ability of a metal to remain liquid at room temperature is fundamentally related to its interatomic bonding and electronic structure. Strong metallic bonds typically lead to higher melting points, as significant energy is required to overcome these strong forces to transition to the liquid phase. Conversely, weaker interatomic forces result in lower melting points.
Several factors influence the strength of metallic bonding:
- Atomic Size and Mass: Larger and heavier atoms generally have weaker metallic bonds due to increased distance between atoms and weaker electrostatic interactions.
- Electron Configuration: The number and arrangement of valence electrons significantly impact the strength of metallic bonding. Metals with loosely held valence electrons tend to exhibit weaker bonds and lower melting points.
- Crystal Structure: The arrangement of atoms in the solid state also affects the strength of the metallic bond and consequently the melting point.
The relatively weak metallic bonding in mercury and its proximity to other low-melting-point metals such as cesium, gallium, and rubidium contribute to their low melting points. The unique electronic structure of mercury, with its loosely held electrons, is a critical factor in its liquid state at room temperature.
Future Possibilities and Research
Research into liquid metals continues to explore their potential applications in various fields, particularly in:
- Liquid Metal Batteries: Offering potentially higher energy density and faster charging compared to traditional battery technologies.
- Heat Transfer Fluids: Used in advanced cooling systems for electronics and other high-heat applications.
- Soft Robotics: The fluidity and malleability of liquid metals make them promising materials for creating flexible and adaptable robots.
- 3D Printing: Liquid metals are being investigated as potential materials for additive manufacturing, allowing for the creation of complex metallic structures.
Overcoming challenges like toxicity (particularly with mercury) and developing more efficient methods for handling and processing these materials will be crucial for realizing the full potential of liquid metals in these emerging technologies.
Conclusion: A Unique State of Matter
The existence of metals that are liquid at room temperature challenges our common understanding of these materials. Mercury, with its unique properties and fascinating history, remains the most prominent example. However, the broader discussion of low-melting-point metals and alloys underscores the complex interplay of atomic structure, bonding, and physical properties that determine a material's phase at a given temperature. Ongoing research continues to uncover new applications and possibilities for these intriguing substances, promising exciting advancements in various technological fields. The study of liquid metals is an active and evolving area of scientific exploration, constantly revealing new insights into the behavior of matter under different conditions. The future of liquid metals holds immense potential, requiring further research and development to fully realize their beneficial applications while mitigating potential risks. Understanding the unique properties and challenges associated with liquid metals is crucial for responsible innovation and technological progress.
Latest Posts
Latest Posts
-
Packing Factor Of Bcc And Fcc
Apr 07, 2025
-
6 Quarts Of Water Is How Many Cups
Apr 07, 2025
-
List The Following Events In The Correct Chronological Order
Apr 07, 2025
-
The Plasma Membrane Of A Muscle Fiber Is Called The
Apr 07, 2025
-
What Is 24 A Multiple Of
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about What Metals Are Liquid At Room Temperature . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
