What Is The Relationship Between Kinetic And Potential Energy
Juapaving
Apr 06, 2025 · 6 min read
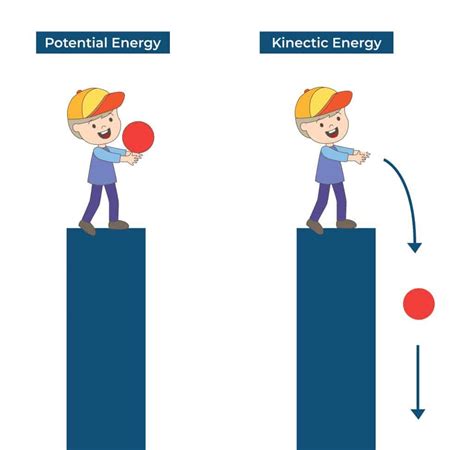
Table of Contents
The Intertwined Dance of Kinetic and Potential Energy: A Deep Dive
The universe is a whirlwind of motion and stillness, a constant interplay between energy in action and energy waiting to be unleashed. At the heart of this dynamic lies the fundamental relationship between kinetic and potential energy – two sides of the same energetic coin. Understanding this relationship is key to unlocking a deeper comprehension of physics, from the simple swing of a pendulum to the complexities of planetary orbits. This article delves into the intricate connection between these two forms of energy, exploring their definitions, examples, and the crucial principle of conservation that governs their transformation.
Defining Kinetic and Potential Energy
Before exploring their relationship, let's establish clear definitions.
Kinetic energy is the energy an object possesses due to its motion. It's the energy of movement, directly proportional to both the mass and the velocity of the object. The faster an object moves and the heavier it is, the more kinetic energy it possesses. Mathematically, kinetic energy (KE) is expressed as:
KE = 1/2 * mv²
where 'm' represents mass and 'v' represents velocity.
Potential energy, on the other hand, is stored energy that has the potential to be converted into kinetic energy. It's energy at rest, waiting to be released. Several types of potential energy exist, including:
-
Gravitational potential energy: This is the energy stored in an object due to its position relative to a gravitational field. The higher an object is above the ground, the greater its gravitational potential energy. Formula: PE = mgh, where 'm' is mass, 'g' is the acceleration due to gravity, and 'h' is height.
-
Elastic potential energy: This is energy stored in a stretched or compressed elastic object, like a spring or a rubber band. The more the object is stretched or compressed, the greater its elastic potential energy.
-
Chemical potential energy: This is energy stored in the chemical bonds of molecules. When these bonds are broken, energy is released, as seen in combustion or digestion.
-
Nuclear potential energy: This is energy stored within the nucleus of an atom, released during nuclear reactions like fission or fusion.
The Interplay: Conversion Between Kinetic and Potential Energy
The crucial aspect of kinetic and potential energy isn't their individual existence but their interchangeability. They are constantly transforming into one another, following the principle of conservation of energy. This principle dictates that energy cannot be created or destroyed, only transformed from one form to another.
Let's examine this conversion through several examples:
1. A Simple Pendulum: A Classic Demonstration
Consider a pendulum swinging back and forth. At its highest point, the pendulum is momentarily motionless. At this apex, its kinetic energy is zero, but its potential energy is at a maximum. As gravity pulls it downwards, the pendulum begins to accelerate, converting potential energy into kinetic energy. At the lowest point of its swing, the pendulum achieves its maximum velocity, meaning its kinetic energy is at a maximum, and its potential energy is at a minimum. This process reverses as the pendulum swings upwards, converting kinetic energy back into potential energy. This continuous cycle of conversion illustrates the dynamic relationship between these two forms of energy.
2. A Roller Coaster: A Thrilling Ride of Energy Transformation
A roller coaster provides another excellent example. At the top of a hill, the coaster car possesses maximum potential energy due to its height. As it plunges downwards, this potential energy is converted into kinetic energy, resulting in increased speed. As the coaster climbs the next hill, the kinetic energy is gradually transformed back into potential energy, slowing the coaster down. The process repeats throughout the ride, with energy constantly shifting between potential and kinetic forms, occasionally losing some to friction (converted into heat).
3. A Ball Thrown into the Air: A Simple yet Profound Example
Throwing a ball straight up showcases the same principle. As you throw the ball upwards, you impart kinetic energy to it. As the ball ascends, this kinetic energy is gradually converted into gravitational potential energy as it gains height, slowing down until it momentarily stops at its highest point. Then, gravity takes over, converting the potential energy back into kinetic energy as the ball falls back to Earth.
4. A Spring: Storing and Releasing Energy
A compressed spring holds elastic potential energy. When released, this potential energy is transformed into kinetic energy, causing the spring to move. The energy conversion continues as the spring oscillates, repeatedly exchanging potential and kinetic energy until it eventually comes to rest (with energy lost to friction).
Factors Affecting the Conversion
Several factors influence the rate and extent of the conversion between kinetic and potential energy:
-
Mass: A heavier object will possess more potential and kinetic energy at any given velocity or height.
-
Velocity: Higher velocity translates to greater kinetic energy.
-
Height: Greater height results in increased gravitational potential energy.
-
Spring Constant: For elastic potential energy, a stiffer spring (higher spring constant) will store more energy for a given compression or extension.
-
Friction and Air Resistance: These forces act as energy dissipators, converting some of the kinetic and potential energy into heat, thus reducing the efficiency of the conversion.
Conservation of Energy: A Fundamental Principle
The conservation of energy principle is paramount in understanding the relationship between kinetic and potential energy. While energy can change forms, the total energy of a system remains constant in the absence of external forces. In our examples, if we neglect friction and air resistance (idealized scenarios), the sum of the kinetic and potential energy at any point in the system's motion remains constant. This constant is often referred to as the mechanical energy of the system.
However, in real-world scenarios, friction and air resistance play significant roles. These forces dissipate energy, converting some of the mechanical energy into heat. Therefore, the total mechanical energy of the system gradually decreases over time, though the total energy of the entire system (including the heat generated) remains constant according to the broader principle of energy conservation.
Beyond the Basics: More Complex Systems
The principles outlined above apply not only to simple systems but also to far more complex ones. Consider:
-
Planetary motion: Planets orbit stars due to a balance between their kinetic energy (orbital velocity) and gravitational potential energy (their distance from the star).
-
Molecular motion: The kinetic energy of molecules determines temperature, while their potential energy contributes to the stability of chemical bonds.
-
Electric circuits: The movement of electrons (kinetic energy) is driven by potential differences (voltage – a form of electrical potential energy).
Conclusion: A Continuous Transformation
The relationship between kinetic and potential energy is a fundamental concept in physics, illustrating the constant interplay between energy in motion and energy at rest. Their continuous transformation, governed by the principle of conservation of energy, underpins countless natural phenomena and technological applications. Understanding this dynamic interplay provides a deeper understanding of the universe's energetic dance, from the smallest subatomic particles to the vast expanse of the cosmos. The more we delve into this intricate relationship, the more we appreciate the elegant simplicity and profound implications of energy conservation. Further exploration into energy transfer, work, and power will reveal even deeper connections and applications of this fundamental concept.
Latest Posts
Latest Posts
-
What Is 24 A Multiple Of
Apr 07, 2025
-
Every Parallelogram Is A Rhombus True Or False
Apr 07, 2025
-
How Many Feet In 11 Yards
Apr 07, 2025
-
Are Rational Numbers Closed Under Multiplication
Apr 07, 2025
-
Least Common Multiple Of 5 And 25
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about What Is The Relationship Between Kinetic And Potential Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
