What Is The Percentage Of Nitrogen In Atmosphere
Juapaving
May 09, 2025 · 6 min read
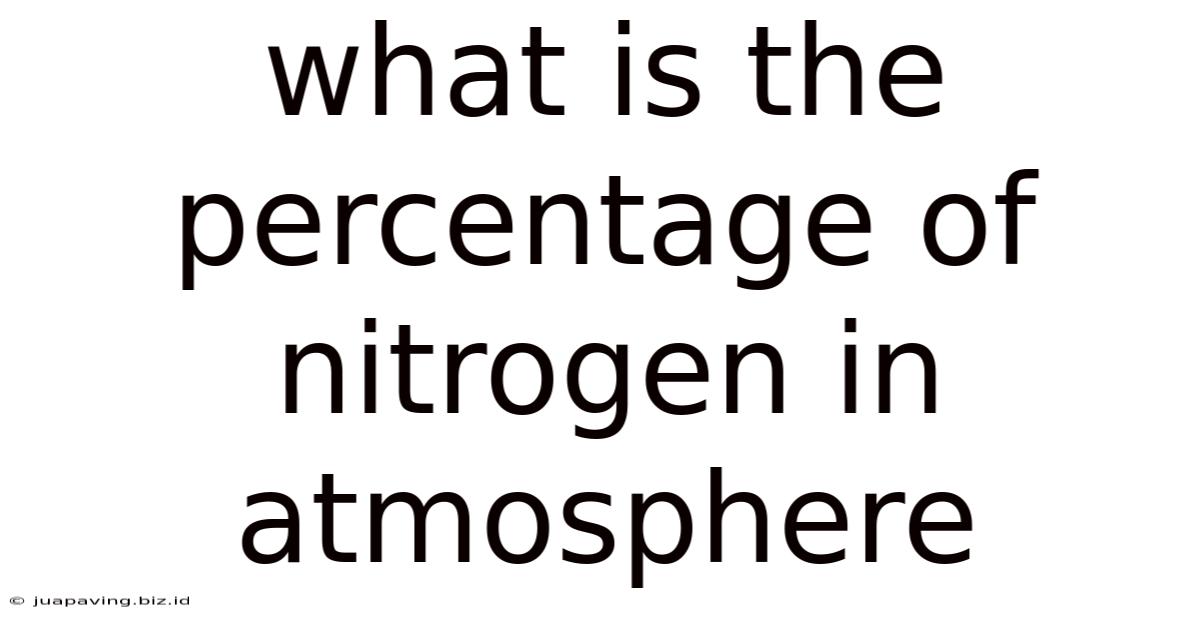
Table of Contents
What is the Percentage of Nitrogen in the Atmosphere?
The Earth's atmosphere is a complex mixture of gases, each playing a crucial role in shaping our planet's environment and supporting life. Among these gases, nitrogen (N₂) stands out as the most abundant component, making up a significant percentage of the air we breathe. Understanding the precise percentage of nitrogen in the atmosphere, and the implications of this abundance, is vital for various scientific fields, including atmospheric science, environmental studies, and biology.
The Dominant Player: Nitrogen's Atmospheric Abundance
Nitrogen comprises approximately 78% of the Earth's atmosphere by volume. This means that out of every 100 molecules of air, roughly 78 are nitrogen molecules. This overwhelming presence makes nitrogen the most abundant gas in our atmosphere, significantly surpassing the concentration of other major components like oxygen.
Understanding the Measurement
The percentage of 78% is an average, and minor fluctuations can occur based on location and altitude. Measurements are typically taken using gas chromatography, a technique that separates and quantifies the different gases in a sample of air. Advanced instruments allow for precise and accurate measurement of atmospheric composition, even detecting trace amounts of less prevalent gases.
Why is it so Abundant?
Nitrogen's dominance isn't accidental. Its chemical inertness plays a key role. Nitrogen molecules (N₂) are incredibly stable due to the strong triple bond between the two nitrogen atoms. This stability means nitrogen is relatively unreactive, meaning it doesn't readily participate in chemical reactions with other atmospheric components. This inertness prevents nitrogen from being readily consumed or transformed into other compounds at the scale necessary to significantly alter its atmospheric concentration.
The Role of Nitrogen in the Atmosphere and Life
While nitrogen's inertness makes it abundant, it's not directly usable by most living organisms in its diatomic form (N₂). This is where the nitrogen cycle comes into play, a crucial biogeochemical cycle that converts nitrogen into usable forms for plants and animals.
The Nitrogen Cycle: From Inert Gas to Life's Building Block
The nitrogen cycle involves several key processes:
-
Nitrogen Fixation: This is the crucial initial step, where specialized bacteria (both free-living and symbiotic) convert atmospheric nitrogen (N₂) into ammonia (NH₃) or other nitrogen-containing compounds. This process breaks the strong triple bond in N₂, making nitrogen available for biological use. Examples of nitrogen-fixing bacteria include those found in the root nodules of leguminous plants (peas, beans, etc.). Industrial nitrogen fixation through the Haber-Bosch process is also vital for producing fertilizers.
-
Nitrification: Ammonia (NH₃) is then converted into nitrites (NO₂⁻) and nitrates (NO₃⁻) by other bacteria. Nitrates are the form of nitrogen most readily absorbed by plants.
-
Assimilation: Plants absorb nitrates from the soil and use them to synthesize proteins, nucleic acids (DNA and RNA), and other vital nitrogen-containing compounds. Animals obtain nitrogen by consuming plants or other animals.
-
Ammonification: When plants and animals die, decomposers break down their organic matter, releasing nitrogen back into the soil as ammonia.
-
Denitrification: Certain bacteria convert nitrates back into atmospheric nitrogen (N₂), completing the cycle and returning nitrogen to its inert state.
Implications of Nitrogen's Abundance
The abundance of nitrogen in the atmosphere has significant implications:
-
Life Support: While not directly usable in its atmospheric form, nitrogen is an essential building block of life, forming part of amino acids, proteins, and nucleic acids. The nitrogen cycle ensures the continuous availability of usable nitrogen for all living organisms.
-
Climate Regulation: While nitrogen itself is not a greenhouse gas, the production and use of nitrogen-based fertilizers significantly impact the climate. The production of fertilizers requires large amounts of energy, contributing to greenhouse gas emissions. Additionally, excess nitrogen from fertilizers can lead to the production of nitrous oxide (N₂O), a potent greenhouse gas with a far greater warming potential than carbon dioxide.
-
Air Quality: Nitrogen oxides (NOx), which are byproducts of combustion processes, contribute to air pollution and acid rain. These pollutants have detrimental effects on human health and the environment.
-
Ocean Acidification: Excessive nitrogen runoff from fertilizers can cause eutrophication in aquatic ecosystems, leading to algal blooms and ultimately, oxygen depletion in the water. This negatively impacts marine life and can even contribute to ocean acidification.
Variations in Nitrogen Percentage: Altitude and Location
While 78% is a good approximation of the average atmospheric nitrogen concentration, minor variations exist based on several factors:
-
Altitude: At higher altitudes, the atmospheric pressure decreases, leading to slightly lower concentrations of all gases, including nitrogen. The percentage remains relatively consistent, but the absolute amount of nitrogen per unit volume is less.
-
Location: Localized variations might occur due to factors like industrial emissions, volcanic activity, or biological processes like nitrogen fixation and denitrification. These variations are generally small compared to the overall atmospheric concentration.
-
Time of Year: Seasonal variations in biological activity can slightly alter local nitrogen concentrations. For instance, increased nitrogen fixation during periods of plant growth could momentarily increase the amount of nitrogen in a specific location. However, these variations are generally localized and short-lived.
The Future of Atmospheric Nitrogen
Human activities have profoundly impacted the nitrogen cycle, altering its natural balance. The widespread use of nitrogen fertilizers has drastically increased the amount of reactive nitrogen in the environment, leading to several environmental problems:
-
Eutrophication: Excess nitrogen in water bodies causes algal blooms, leading to oxygen depletion and harming aquatic life.
-
Acid Rain: Nitrogen oxides contribute to acid rain, damaging forests and aquatic ecosystems.
-
Greenhouse Gas Emissions: Nitrous oxide (N₂O), a potent greenhouse gas, is a byproduct of fertilizer use and other human activities.
-
Ozone Depletion: While less significant than the effects of chlorofluorocarbons, nitrogen oxides can contribute to ozone depletion in the stratosphere.
Scientists are actively researching sustainable ways to manage nitrogen use in agriculture and other industries. This includes exploring alternative fertilizers, improving fertilizer application techniques to minimize losses, and developing strategies to reduce nitrogen oxide emissions. Maintaining a healthy nitrogen cycle is crucial for protecting environmental health and ensuring the long-term availability of this essential element for life.
Conclusion: A Vital Component of Our Atmosphere
Nitrogen's 78% abundance in the Earth's atmosphere is a fundamental aspect of our planet's environment and the sustenance of life. While its inert nature prevents it from being directly usable by most organisms, the nitrogen cycle plays a vital role in converting it into a form accessible to plants and animals. However, human activities have significantly impacted the nitrogen cycle, highlighting the need for responsible management of this crucial element and mitigating the negative consequences of excessive nitrogen in the environment. Further research and sustainable practices are essential to ensure the healthy functioning of the nitrogen cycle for generations to come. Understanding the percentage of nitrogen and its intricate role in atmospheric chemistry and the biosphere remains a critical area of scientific study.
Latest Posts
Latest Posts
-
Five Letter Words Starting With Tho
May 09, 2025
-
How To Calculate Radius Of Gyration
May 09, 2025
-
Which Of The Following Is A Pair Of Vertical Angles
May 09, 2025
-
What Are The Two Types Of Interference
May 09, 2025
-
Which Statement Best Describes Human Eye Color
May 09, 2025
Related Post
Thank you for visiting our website which covers about What Is The Percentage Of Nitrogen In Atmosphere . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.