What Is The Optimal Ph For Pepsin
Juapaving
Apr 08, 2025 · 5 min read
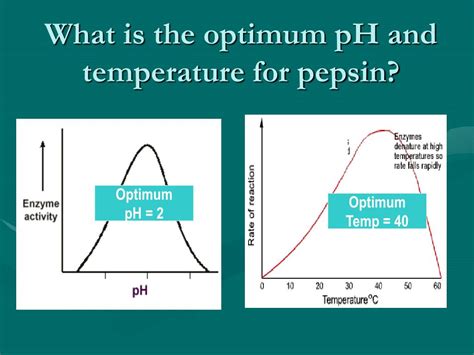
Table of Contents
What is the Optimal pH for Pepsin? Understanding the Enzyme's Role in Digestion
Pepsin, a crucial enzyme in the human digestive system, plays a vital role in protein breakdown. Understanding its optimal pH is essential to grasping its function and the overall process of digestion. This article delves deep into the optimal pH for pepsin activity, exploring the reasons behind this specificity, its implications for health, and related factors influencing its performance.
The Role of Pepsin in Digestion
Pepsin is an endopeptidase, meaning it cleaves peptide bonds within a protein molecule, breaking down large proteins into smaller peptides. This initial breakdown is critical, paving the way for further digestion in the small intestine. Unlike many other enzymes, pepsin operates most effectively in a highly acidic environment. This acidic environment is crucial for its activation and optimal functioning.
The Gastric Environment: A Perfect Storm for Pepsin
The stomach, with its extremely acidic environment, provides the perfect habitat for pepsin. The stomach lining secretes hydrochloric acid (HCl), creating a pH typically ranging from 1.5 to 3.5. This low pH is not only essential for activating pepsinogen (pepsin's inactive precursor) but also for maintaining its optimal activity.
Optimal pH for Pepsin: The Sweet Spot for Protein Digestion
The optimal pH for pepsin is remarkably low, sitting around pH 1.5 to 2.0. At this pH, the enzyme's three-dimensional structure—its tertiary structure—is ideally configured to bind to and effectively cleave peptide bonds in proteins. Any significant deviation from this optimal range results in decreased activity.
Understanding the pH-Activity Curve
The relationship between pH and pepsin activity can be graphically represented by a pH-activity curve. This curve demonstrates that pepsin activity increases as the pH decreases from neutral (pH 7) towards its optimal range. However, beyond pH 1.5, the activity begins to decline, indicating that extreme acidity can also negatively impact pepsin's function.
The Importance of the Low pH Environment
The low pH environment in the stomach serves multiple purposes in relation to pepsin:
-
Pepsinogen Activation: Pepsin is initially synthesized and secreted as an inactive precursor called pepsinogen. The acidic environment in the stomach triggers a conformational change in pepsinogen, converting it into its active form, pepsin. This autocatalytic process ensures that pepsin only becomes active within the stomach, preventing self-digestion of the gastric mucosa.
-
Optimal Enzyme Conformation: The acidic pH maintains the correct three-dimensional structure of the pepsin enzyme, crucial for its catalytic activity. Changes in pH can alter this structure, reducing its effectiveness in cleaving peptide bonds.
-
Protein Denaturation: The low pH also contributes to the denaturation of ingested proteins. Denaturation unfolds the protein structure, making the peptide bonds more accessible to pepsin's active site. This enhances the efficiency of protein digestion.
Factors Affecting Pepsin Activity Beyond pH
While pH is the most critical factor, other factors also influence pepsin's activity:
-
Temperature: Like all enzymes, pepsin's activity is temperature-dependent. It exhibits optimal activity at around 37°C (body temperature). Higher temperatures can denature the enzyme, reducing its activity, while lower temperatures slow down the reaction rate.
-
Substrate Concentration: The concentration of the protein substrate also affects pepsin's activity. Increased substrate concentration initially leads to increased activity, up to a point of saturation where all enzyme molecules are occupied.
-
Enzyme Concentration: Similarly, the concentration of pepsin itself impacts its activity. Higher concentrations lead to faster digestion, but again, there's a saturation point.
-
Inhibitors: Certain substances can inhibit pepsin's activity. For instance, pepstatin A, a naturally occurring compound, is a potent pepsin inhibitor. This is relevant in research settings and certain medical contexts.
Implications of Suboptimal pH for Pepsin Activity
Deviations from the optimal pH range for pepsin can have significant consequences:
-
Hypochlorhydria: This condition, characterized by insufficient stomach acid production, leads to a higher-than-optimal stomach pH. This reduces pepsin activity, hindering protein digestion and potentially leading to digestive discomfort, nutrient deficiencies, and increased risk of infection.
-
Achlorhydria: This condition, where the stomach produces virtually no acid, results in severely impaired pepsin activity. It is associated with various health issues, including anemia due to impaired vitamin B12 absorption.
-
Hyperchlorhydria: Conversely, excessively high stomach acid levels (though less common) can also disrupt the optimal pH range for pepsin, although the effect is less dramatic than hypochlorhydria. Prolonged hyperchlorhydria can damage the stomach lining.
-
Gastroesophageal Reflux Disease (GERD): In GERD, stomach acid (including pepsin) refluxes into the esophagus, damaging its delicate lining. The acidic pepsin contributes to esophageal irritation and inflammation.
Pepsin and Related Enzymes: A Collaborative Effort
Pepsin's action is not isolated; it works in conjunction with other digestive enzymes and factors to ensure complete protein digestion. The subsequent steps in protein digestion involve other enzymes like trypsin, chymotrypsin, and carboxypeptidase in the small intestine, working at a more neutral pH.
Research and Future Directions
Ongoing research continues to refine our understanding of pepsin, its optimal pH, and its intricate role in human digestion. Studies explore pepsin's involvement in various health conditions, including inflammatory bowel disease (IBD) and cancer. Further research may lead to the development of novel therapies targeting pepsin activity for treating specific digestive disorders.
Conclusion: The Importance of Optimal pH for Pepsin Function
The optimal pH for pepsin, around 1.5 to 2.0, is critical for its proper function. This low pH is essential for its activation, maintaining its three-dimensional structure, and facilitating effective protein breakdown. Understanding the factors influencing pepsin activity and the implications of deviations from its optimal pH is key to maintaining optimal digestive health. Maintaining a healthy stomach environment, with appropriate acid production, is crucial for ensuring the efficient action of pepsin and the overall process of protein digestion. Disruptions in this delicate balance can lead to various digestive problems, highlighting the pivotal role of pH in this vital physiological process. The continued investigation into pepsin's behavior and its interaction with the broader digestive system promises further insights into human health and well-being.
Latest Posts
Latest Posts
-
What Do You Call A Group Of Lions
Apr 08, 2025
-
Whats The Lcm Of 9 And 12
Apr 08, 2025
-
Which Is Not One Of The Five Pillars Of Islam
Apr 08, 2025
-
How Many Feet Is 65 In
Apr 08, 2025
-
5 Letter Words Beginning With Ae
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about What Is The Optimal Ph For Pepsin . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.