What Is The Number Of Valence Electrons In Phosphorus
Juapaving
Apr 06, 2025 · 5 min read
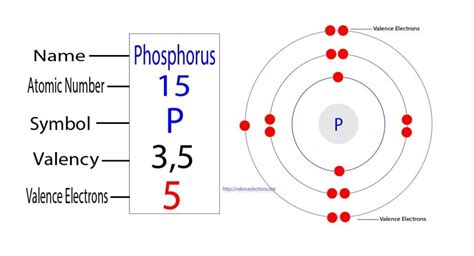
Table of Contents
What is the Number of Valence Electrons in Phosphorus? A Deep Dive into Atomic Structure and Chemical Bonding
Phosphorus, a vital element for life, plays a crucial role in various biological processes and industrial applications. Understanding its chemical behavior necessitates a firm grasp of its electronic structure, particularly the number of valence electrons it possesses. This article delves into the intricacies of phosphorus's atomic structure, explaining how to determine its valence electrons and exploring the implications of this number on its reactivity and bonding characteristics. We will also touch upon the different allotropes of phosphorus and how their structures influence their properties.
Understanding Valence Electrons: The Key to Chemical Reactivity
Before focusing specifically on phosphorus, let's establish a foundational understanding of valence electrons. Valence electrons are the electrons located in the outermost shell of an atom. These electrons are the primary participants in chemical bonding, determining an element's reactivity and the types of bonds it can form (ionic, covalent, metallic). The number of valence electrons directly influences an atom's ability to gain, lose, or share electrons to achieve a stable electron configuration, often referred to as a filled outer shell, typically resembling a noble gas configuration. This stable configuration is energetically favorable and drives chemical reactions.
Determining the Number of Valence Electrons in Phosphorus
Phosphorus (P) has an atomic number of 15, meaning it possesses 15 protons and 15 electrons in a neutral atom. To determine its valence electrons, we need to examine its electron configuration. Using the Aufbau principle and Hund's rule, we can systematically fill the electron orbitals:
- 1s² 2s² 2p⁶ 3s² 3p³
This electron configuration reveals the distribution of electrons across different energy levels (shells). The outermost shell is the third shell (n=3), which contains both the 3s and 3p orbitals. The 3s subshell contains 2 electrons, and the 3p subshell contains 3 electrons.
Therefore, phosphorus has a total of five valence electrons (2 from the 3s and 3 from the 3p subshells).
Visualizing Phosphorus's Electronic Structure
Visualizing the electronic structure can enhance understanding. The Bohr model, though simplistic, provides a helpful visual representation. It shows the 15 electrons arranged in shells around the nucleus: two in the first shell, eight in the second shell, and five in the third shell (the valence shell).
The Significance of Five Valence Electrons
The presence of five valence electrons is crucial in determining phosphorus's chemical behavior. To achieve a stable octet (eight electrons in its outermost shell), a phosphorus atom can:
-
Gain three electrons: This leads to the formation of the phosphide anion (P³⁻), which has a stable electron configuration similar to argon. This is observed in ionic compounds formed with highly electropositive elements such as alkali metals and alkaline earth metals.
-
Share three electrons: This results in the formation of covalent bonds, where phosphorus shares electrons with other atoms. This is the most common type of bonding observed in phosphorus compounds. Phosphorus can form single, double, and even triple bonds, depending on the other atoms involved.
-
Share five electrons: Although less common, phosphorus can also share all five of its valence electrons, particularly in compounds with highly electronegative atoms like oxygen and halogens. This can lead to the formation of molecules with expanded octets (more than eight electrons in the valence shell).
Phosphorus Allotropes: Structure and Properties
Phosphorus exhibits several allotropic forms, each with distinct physical and chemical properties due to differences in their atomic arrangements. The most common allotropes are white phosphorus, red phosphorus, and black phosphorus. The number of valence electrons remains consistent (five) across all allotropes, but the way these electrons are involved in bonding differs significantly.
White Phosphorus
White phosphorus (P₄) is a highly reactive and toxic substance. Its structure consists of four phosphorus atoms arranged in a tetrahedral structure. Each phosphorus atom is bonded to three other phosphorus atoms through single covalent bonds, utilizing three of its five valence electrons. The remaining two electrons on each phosphorus atom are considered lone pairs. This unique structure makes white phosphorus highly reactive and prone to oxidation, often igniting spontaneously in air.
Red Phosphorus
Red phosphorus is a less reactive and less toxic allotrope compared to white phosphorus. Its structure is polymeric, involving a complex network of phosphorus atoms linked together. This polymeric structure significantly reduces the reactivity compared to the discrete tetrahedral structure of white phosphorus. The bonding within red phosphorus is still covalent, but the network structure limits its ability to easily form new bonds.
Black Phosphorus
Black phosphorus is the most stable and least reactive allotrope of phosphorus. It possesses a layered structure similar to graphite, with phosphorus atoms arranged in puckered layers. These layers are held together by weak van der Waals forces. The bonding within each layer is covalent, involving all five valence electrons, resulting in a more stable configuration compared to white and red phosphorus.
Applications of Phosphorus and its Compounds
The diverse properties of phosphorus and its compounds lead to a wide range of applications in various fields:
-
Fertilizers: Phosphate-containing fertilizers are crucial for plant growth, supplying essential phosphorus nutrients. This makes phosphorus compounds indispensable in agriculture.
-
Detergents: Phosphates were widely used in detergents as builders, but their use has been reduced due to environmental concerns related to eutrophication.
-
Matches: White phosphorus was historically used in the production of matches, although its toxicity led to its replacement with safer alternatives.
-
Metallurgy: Phosphorus compounds are used in the production of certain alloys and in deoxidizing metals.
-
Biological systems: Phosphorus is an essential element in biological molecules like DNA, RNA, and ATP, crucial for energy transfer and genetic information storage.
Conclusion: The Importance of Valence Electrons in Understanding Phosphorus
The five valence electrons of phosphorus are the key to understanding its chemical behavior and the diversity of its compounds. This number dictates its reactivity, the types of bonds it forms, and the various allotropic forms it exhibits. Understanding valence electrons is fundamental to comprehending the properties and applications of phosphorus in diverse fields, from agriculture to medicine. Its unique electronic structure and resulting chemical reactivity make it an element of critical importance in both natural and man-made systems. The information provided here offers a comprehensive exploration of the valence electron count of phosphorus, its implications, and related concepts. Further research into specific phosphorus compounds and their applications can provide even deeper insights into the fascinating world of this essential element. The interplay of atomic structure, bonding characteristics, and allotropic variations ultimately defines the unique roles phosphorus plays in our world.
Latest Posts
Latest Posts
-
What Is One Of The Basic Principles Of Democracy
Apr 08, 2025
-
100 100 Divided By 100 100 Is Equal To 2
Apr 08, 2025
-
The Amount Of Water Vapor In The Air Is Called
Apr 08, 2025
-
Common Multiples Of 3 And 8
Apr 08, 2025
-
What Is Group Of Kangaroos Called
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about What Is The Number Of Valence Electrons In Phosphorus . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.