What Is The Molar Mass Of Mg No3 2
Juapaving
May 10, 2025 · 5 min read
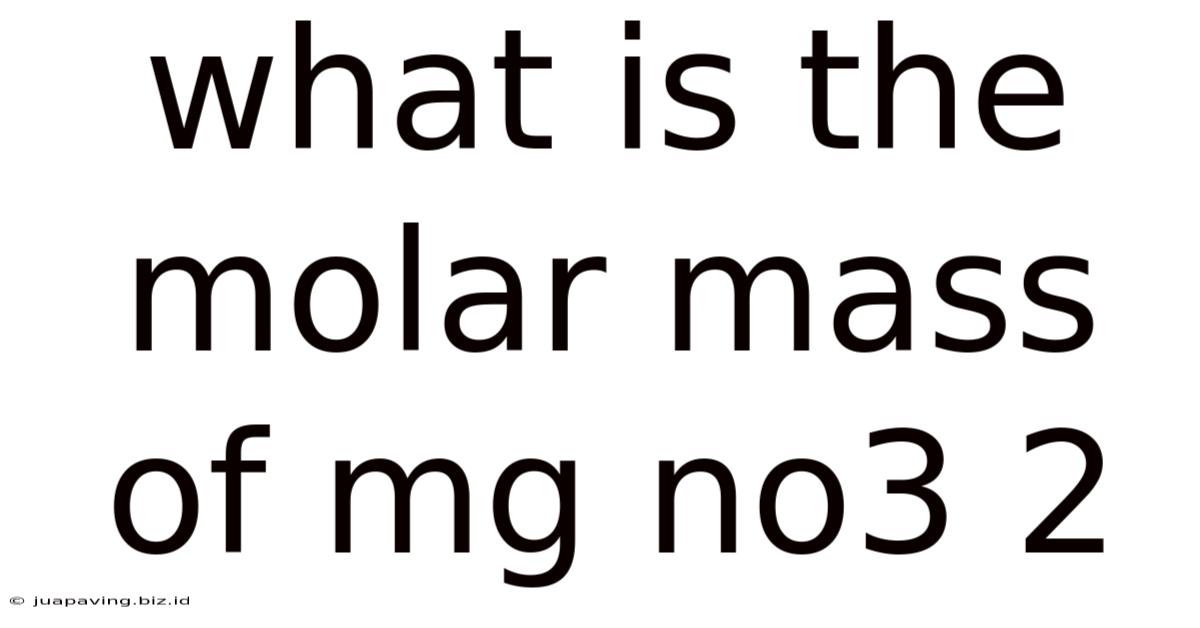
Table of Contents
What is the Molar Mass of Mg(NO₃)₂? A Comprehensive Guide
Determining the molar mass of a compound is a fundamental concept in chemistry, crucial for various calculations and analyses. This article will delve into the process of calculating the molar mass of magnesium nitrate, Mg(NO₃)₂, explaining the underlying principles and providing a step-by-step guide. We'll also explore the significance of molar mass in different chemical contexts and address common misconceptions.
Understanding Molar Mass
Molar mass, expressed in grams per mole (g/mol), represents the mass of one mole of a substance. A mole is a unit of measurement in chemistry that represents Avogadro's number (approximately 6.022 x 10²³) of particles, whether they are atoms, molecules, ions, or formula units. Essentially, the molar mass tells us the mass of 6.022 x 10²³ particles of a given substance.
To calculate the molar mass of a compound, we need to consider the atomic masses of its constituent elements and their respective quantities within the chemical formula. These atomic masses are typically found on the periodic table of elements.
Calculating the Molar Mass of Mg(NO₃)₂
Magnesium nitrate, Mg(NO₃)₂, is an ionic compound consisting of magnesium cations (Mg²⁺) and nitrate anions (NO₃⁻). To calculate its molar mass, we will follow these steps:
Step 1: Identify the Elements and Their Atomic Masses
The chemical formula Mg(NO₃)₂ indicates that the compound contains:
- Magnesium (Mg): 1 atom
- Nitrogen (N): 2 atoms (because of the subscript 2 outside the parenthesis)
- Oxygen (O): 6 atoms (2 x 3 = 6, due to the subscript 2 and 3)
Now, we consult the periodic table to find the atomic masses of these elements. The values may vary slightly depending on the source, but for our calculation, we'll use the following approximate atomic masses:
- Mg: 24.31 g/mol
- N: 14.01 g/mol
- O: 16.00 g/mol
Step 2: Calculate the Contribution of Each Element
Next, we multiply the atomic mass of each element by the number of atoms of that element present in the compound:
- Magnesium (Mg): 1 atom × 24.31 g/mol = 24.31 g/mol
- Nitrogen (N): 2 atoms × 14.01 g/mol = 28.02 g/mol
- Oxygen (O): 6 atoms × 16.00 g/mol = 96.00 g/mol
Step 3: Sum the Contributions
Finally, we add up the contributions of each element to obtain the molar mass of Mg(NO₃)₂:
24.31 g/mol + 28.02 g/mol + 96.00 g/mol = 148.33 g/mol
Therefore, the molar mass of Mg(NO₃)₂ is approximately 148.33 g/mol.
Significance of Molar Mass
The molar mass of Mg(NO₃)₂ and other compounds plays a vital role in various chemical calculations and applications, including:
1. Stoichiometry
Stoichiometry involves the quantitative relationships between reactants and products in chemical reactions. Knowing the molar mass allows us to convert between mass and moles, which is essential for determining the amounts of reactants needed or products formed in a reaction. For example, if you need to prepare a specific number of moles of Mg(NO₃)₂, you can use its molar mass to calculate the required mass.
2. Solution Preparation
Molar mass is crucial for preparing solutions of a specific concentration, such as molarity (moles of solute per liter of solution). To make a 1 M solution of Mg(NO₃)₂, you'd need to dissolve 148.33 grams of Mg(NO₃)₂ in enough solvent to make one liter of solution.
3. Gravimetric Analysis
Gravimetric analysis is a quantitative method for determining the amount of a substance by measuring its mass. Molar mass is used to convert the measured mass of a precipitate or other product to the amount of the original analyte.
4. Gas Law Calculations
For gaseous substances, molar mass is used in conjunction with gas laws (e.g., Ideal Gas Law) to relate the mass, volume, pressure, and temperature of the gas.
5. Determining Empirical and Molecular Formulas
Molar mass is crucial in determining the empirical and molecular formulas of unknown compounds. The empirical formula gives the simplest whole-number ratio of atoms in a compound, while the molecular formula gives the actual number of atoms of each element. Molar mass helps bridge the gap between these two formulas.
Addressing Common Misconceptions
Several misconceptions surround molar mass calculations. Let's clarify some of them:
-
Ignoring Subscripts and Parentheses: It is crucial to account for all subscripts and parentheses in the chemical formula when calculating the molar mass. Failure to do so will lead to an incorrect result. For example, the 2 outside the parentheses in Mg(NO₃)₂ significantly impacts the number of nitrogen and oxygen atoms.
-
Using Incorrect Atomic Masses: Always use the most up-to-date atomic masses from a reliable source, such as a current periodic table. Slight variations in atomic masses from different sources can lead to minor discrepancies in the final molar mass.
-
Rounding Errors: While it's acceptable to round atomic masses to a reasonable number of significant figures during the calculation, avoid excessive rounding, which can accumulate errors and affect the accuracy of the final result.
-
Confusing Atomic Mass with Molar Mass: Atomic mass refers to the mass of a single atom of an element, while molar mass refers to the mass of one mole of a substance. They are related but not interchangeable.
Conclusion
Calculating the molar mass of Mg(NO₃)₂, or any compound for that matter, is a fundamental skill in chemistry. This process involves identifying the elements, determining their atomic masses from the periodic table, considering the stoichiometry of the compound, and summing the contributions of each element to obtain the overall molar mass. Understanding molar mass is critical for various chemical calculations and applications, spanning stoichiometry, solution preparation, gravimetric analysis, and gas law calculations. By mastering this concept and avoiding common pitfalls, you'll build a strong foundation for more advanced chemical studies. Remember to always double-check your calculations and use reliable sources for atomic masses to ensure accuracy. The precise molar mass of Mg(NO₃)₂, as calculated using the latest atomic mass data, might slightly vary, but the method presented here provides a clear and accurate approach.
Latest Posts
Latest Posts
-
Illustration Of Newtons Second Law Of Motion
May 10, 2025
-
What Is 5 Out Of 8 As A Percentage
May 10, 2025
-
118 In Is How Many Feet
May 10, 2025
-
What Is The Electron Configuration For Ti
May 10, 2025
-
Greatest Common Factor Of 28 And 32
May 10, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molar Mass Of Mg No3 2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.