What Is The Molar Mass Of Ch3cooh
Juapaving
May 09, 2025 · 4 min read
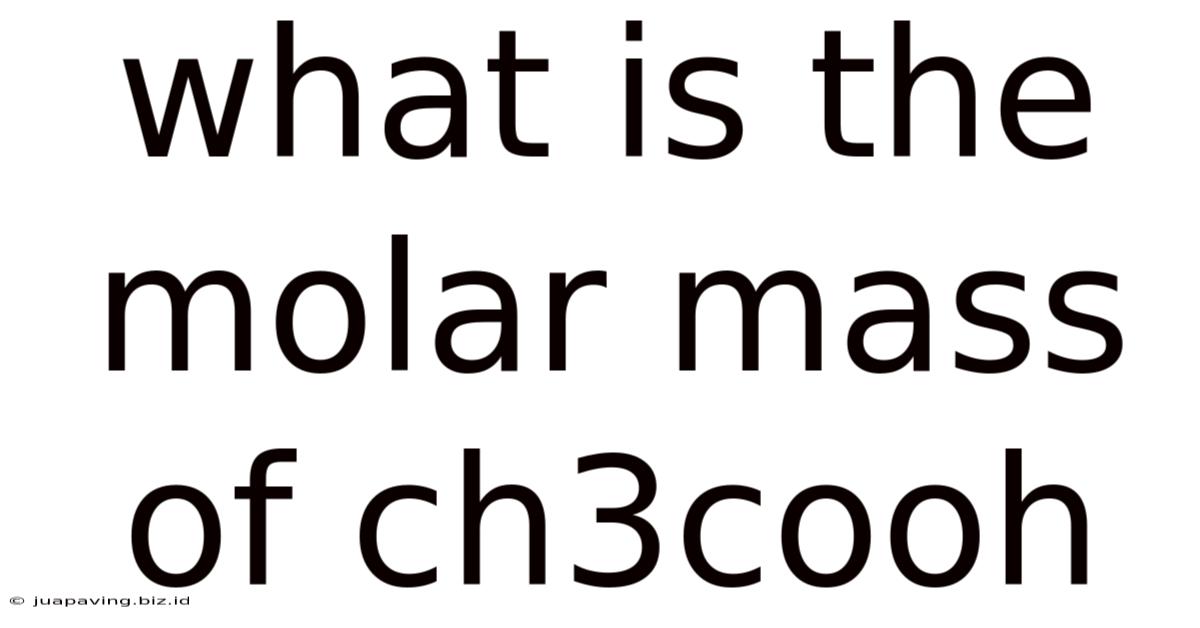
Table of Contents
What is the Molar Mass of CH3COOH? A Deep Dive into Acetic Acid
Acetic acid, also known as ethanoic acid, is a ubiquitous organic compound with the chemical formula CH₃COOH. It's the main component of vinegar, lending its characteristic sour taste and pungent smell. Understanding its molar mass is crucial in various applications, from stoichiometric calculations in chemistry to industrial processes involving acetic acid production and utilization. This comprehensive guide will delve into the determination of the molar mass of CH₃COOH, exploring the underlying principles and providing practical examples.
Understanding Molar Mass
Before we calculate the molar mass of CH₃COOH, let's establish a fundamental understanding of what molar mass represents. Molar mass is the mass of one mole of a substance. A mole is a fundamental unit in chemistry, defined as the amount of a substance that contains Avogadro's number (approximately 6.022 x 10²³) of particles (atoms, molecules, ions, etc.). The molar mass is expressed in grams per mole (g/mol).
The molar mass of an element is numerically equal to its atomic weight (relative atomic mass) found on the periodic table. For example, the atomic weight of carbon (C) is approximately 12.01, so its molar mass is 12.01 g/mol. For compounds, the molar mass is the sum of the molar masses of all the atoms in the molecule.
Calculating the Molar Mass of CH₃COOH
To calculate the molar mass of CH₃COOH (acetic acid), we need to consider the molar masses of its constituent elements: carbon (C), hydrogen (H), and oxygen (O). From the periodic table, we obtain the following approximate molar masses:
- Carbon (C): 12.01 g/mol
- Hydrogen (H): 1.01 g/mol
- Oxygen (O): 16.00 g/mol
Now let's break down the CH₃COOH molecule:
- 2 Carbon atoms (C): 2 * 12.01 g/mol = 24.02 g/mol
- 4 Hydrogen atoms (H): 4 * 1.01 g/mol = 4.04 g/mol
- 2 Oxygen atoms (O): 2 * 16.00 g/mol = 32.00 g/mol
To find the total molar mass of CH₃COOH, we sum the molar masses of all the atoms:
24.02 g/mol + 4.04 g/mol + 32.00 g/mol = 60.06 g/mol
Therefore, the molar mass of CH₃COOH (acetic acid) is approximately 60.06 g/mol.
Applications of Molar Mass of CH₃COOH
The molar mass of CH₃COOH is crucial in numerous chemical calculations and applications:
1. Stoichiometry
In stoichiometric calculations, the molar mass is used to convert between mass and moles. For example, if you need to determine the number of moles of acetic acid in a given mass of vinegar, you would use the molar mass (60.06 g/mol) as a conversion factor.
2. Solution Preparation
When preparing solutions of acetic acid, the molar mass is essential for calculating the required mass to achieve a specific molar concentration (e.g., molarity).
3. Titrations
In acid-base titrations, the molar mass is used to calculate the concentration of an unknown acetic acid solution based on the volume and concentration of the titrant.
4. Industrial Processes
In industrial settings where acetic acid is produced or used, precise knowledge of its molar mass is crucial for optimizing reaction yields, controlling reaction conditions, and ensuring product quality. The molar mass aids in calculating the quantities of reactants and products in industrial-scale reactions.
5. Chemical Analysis
Determining the purity of acetic acid samples often involves analyzing the mass of acetic acid present. Using the molar mass enables accurate calculation of the percentage purity.
Factors Affecting Molar Mass Calculations
While the molar mass calculation presented above uses standard atomic weights, slight variations can occur due to isotopic abundances. Isotopes are atoms of the same element with different numbers of neutrons. The naturally occurring isotopes of carbon, hydrogen, and oxygen have slightly different masses. However, the variations are usually negligible for most practical purposes, and the calculated molar mass of 60.06 g/mol remains a highly accurate approximation.
Beyond the Basics: Isotopic Effects and High Precision
For extremely high-precision calculations, isotopic abundances must be considered. The standard atomic weights used in the previous calculation are weighted averages of the masses of the different isotopes of each element. If the exact isotopic composition of the acetic acid sample is known, a more precise molar mass calculation can be performed using the individual isotopic masses instead of the average atomic weights. However, this level of precision is generally unnecessary for most applications.
Conclusion: The Importance of Precision in Chemical Calculations
The molar mass of CH₃COOH, approximately 60.06 g/mol, is a fundamental value in various chemical calculations and applications involving acetic acid. While the approximation is highly accurate for most purposes, understanding the potential influence of isotopic variations is crucial for high-precision scientific endeavors. Accurate determination of molar mass ensures the reliability and precision of numerous chemical analyses, industrial processes, and research findings. This understanding forms a cornerstone of accurate quantitative chemistry and facilitates various applications across diverse fields. Mastering the calculation of molar mass, therefore, is an essential skill for any student or professional working with chemical compounds.
Latest Posts
Latest Posts
-
What The Freezing Point Of Water In Fahrenheit
May 10, 2025
-
No Of Base Pairs In E Coli
May 10, 2025
-
How To Calculate Probability Of Event
May 10, 2025
-
Which Of The Following Substances Is An Element
May 10, 2025
-
Salt Dissolving In Water Is A Physical Change
May 10, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molar Mass Of Ch3cooh . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.