What Is The Molar Mass Of Calcium Nitrate
Juapaving
Mar 16, 2025 · 5 min read
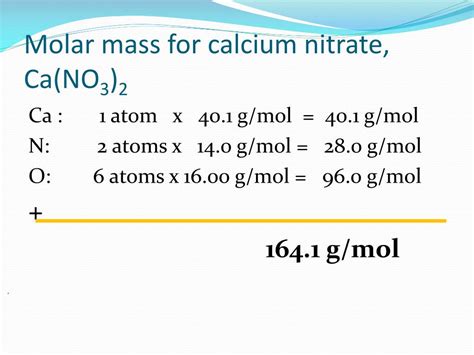
Table of Contents
What is the Molar Mass of Calcium Nitrate? A Deep Dive into Chemical Calculations
Understanding molar mass is fundamental to various chemical calculations, including stoichiometry, solution preparation, and determining the concentration of substances. This article delves into the calculation of the molar mass of calcium nitrate (Ca(NO₃)₂), explaining the process step-by-step and highlighting its importance in chemistry. We'll also explore related concepts and practical applications.
Understanding Molar Mass
Molar mass, also known as molecular weight, represents the mass of one mole of a substance. A mole is a fundamental unit in chemistry, defined as the amount of a substance containing Avogadro's number (approximately 6.022 x 10²³) of particles (atoms, molecules, ions, etc.). The molar mass is expressed in grams per mole (g/mol). It's a crucial tool for converting between mass and moles, allowing chemists to perform accurate calculations in various experiments and analyses.
Calculating the Molar Mass of Calcium Nitrate (Ca(NO₃)₂)
Calcium nitrate, Ca(NO₃)₂, is an ionic compound composed of calcium (Ca²⁺) cations and nitrate (NO₃⁻) anions. To calculate its molar mass, we need to consider the atomic masses of each element present in the compound. These atomic masses are typically found on the periodic table.
Step 1: Identify the elements and their atomic masses:
- Calcium (Ca): Atomic mass ≈ 40.08 g/mol
- Nitrogen (N): Atomic mass ≈ 14.01 g/mol
- Oxygen (O): Atomic mass ≈ 16.00 g/mol
Step 2: Determine the number of atoms of each element in the formula:
- Calcium (Ca): 1 atom
- Nitrogen (N): 2 atoms (from the subscript 2 outside the parentheses)
- Oxygen (O): 6 atoms (2 atoms per nitrate ion × 3 nitrate ions)
Step 3: Calculate the molar mass:
Molar mass of Ca(NO₃)₂ = (1 × atomic mass of Ca) + (2 × atomic mass of N) + (6 × atomic mass of O)
Molar mass of Ca(NO₃)₂ = (1 × 40.08 g/mol) + (2 × 14.01 g/mol) + (6 × 16.00 g/mol)
Molar mass of Ca(NO₃)₂ = 40.08 g/mol + 28.02 g/mol + 96.00 g/mol
Molar mass of Ca(NO₃)₂ ≈ 164.10 g/mol
Therefore, the molar mass of calcium nitrate is approximately 164.10 grams per mole. This value is essential for various stoichiometric calculations involving calcium nitrate.
Significance of Molar Mass in Chemical Calculations
The molar mass of calcium nitrate, and other compounds, is crucial for numerous chemical calculations:
-
Stoichiometry: Molar mass allows for the conversion between grams and moles, essential for solving stoichiometry problems, determining limiting reactants, and calculating theoretical yields in chemical reactions. For example, if we know the mass of calcium nitrate used in a reaction, we can use its molar mass to determine the number of moles involved.
-
Solution Preparation: When preparing solutions of a specific concentration (e.g., molarity), the molar mass is vital for accurately weighing the required amount of solute. Molarity (M) is defined as moles of solute per liter of solution. Knowing the molar mass allows for the calculation of the mass required to achieve a specific molarity.
-
Titration Calculations: In titrations, where a solution of known concentration is used to determine the concentration of an unknown solution, the molar mass of the reactants is necessary to calculate the unknown concentration from titration data.
-
Determining the Empirical Formula: Molar mass, combined with elemental analysis data (percentage composition of each element), allows for the determination of the empirical formula (simplest whole-number ratio of atoms in a compound).
-
Understanding Chemical Properties: The molar mass contributes to understanding a compound's chemical properties. For instance, the molar mass can be related to factors like boiling point, freezing point, and solubility.
Practical Applications of Calcium Nitrate
Calcium nitrate finds widespread applications in various fields due to its properties as a soluble calcium and nitrogen source:
-
Agriculture: It's a common nitrogen fertilizer, providing essential nutrients for plant growth. Its water solubility facilitates easy absorption by plants. The precise amount of fertilizer needed can be calculated using its molar mass.
-
Food Industry: Calcium nitrate is used as a food preservative, and in some cases, as a curing agent in processed meats.
-
Industrial Applications: It finds use in various industrial processes, including cement production and water treatment.
-
Laboratory Use: In laboratories, it's used in experiments and research involving calcium and nitrate ions. Accurate calculations using its molar mass are critical for these applications.
Potential Sources of Error in Molar Mass Calculations
While calculating molar mass is relatively straightforward, there are potential sources of error:
-
Inaccurate Atomic Masses: Using outdated or imprecise atomic masses from the periodic table can lead to inaccuracies in the calculated molar mass. Using a reliable and up-to-date periodic table is crucial.
-
Incorrect Formula: Errors in writing the chemical formula of the compound will result in incorrect molar mass calculation. Double-checking the formula is essential.
-
Calculation Mistakes: Simple arithmetic errors during the calculation can affect the accuracy of the result. Careful and meticulous calculations are necessary.
-
Significant Figures: Paying attention to significant figures is important to avoid overstating the precision of the calculated molar mass. The final answer should reflect the precision of the input values.
Conclusion
The molar mass of calcium nitrate, approximately 164.10 g/mol, is a fundamental value in chemistry, essential for accurate stoichiometric calculations, solution preparation, and understanding the compound's behavior in various applications. Understanding the calculation process and being mindful of potential sources of error are vital for obtaining reliable results in chemical experiments and analyses. The significance of molar mass extends far beyond simple calculations; it's a cornerstone of quantitative chemistry and is crucial for various practical applications across various scientific disciplines and industries. Accurate calculation and a thorough understanding of its implications contribute to the success and reliability of many scientific and industrial endeavors.
Latest Posts
Related Post
Thank you for visiting our website which covers about What Is The Molar Mass Of Calcium Nitrate . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
