What Is The Formula Of Phosphorus Pentachloride
Juapaving
Mar 13, 2025 · 5 min read
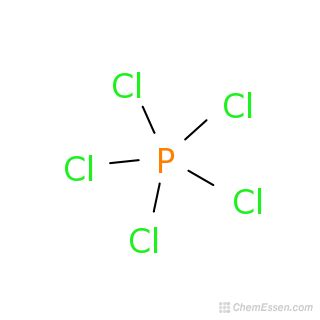
Table of Contents
What is the Formula of Phosphorus Pentachloride? A Deep Dive into its Structure, Properties, and Reactions
Phosphorus pentachloride (PCl₅) is a crucial chemical compound with a wide array of applications in various industries. Understanding its formula, structure, properties, and reactions is essential for anyone working with or studying this versatile substance. This comprehensive article will delve into all these aspects, providing a detailed exploration of phosphorus pentachloride.
Understanding the Formula: PCl₅
The formula PCl₅ itself concisely communicates the composition of phosphorus pentachloride. It indicates that one molecule of this compound contains:
-
One phosphorus (P) atom: Phosphorus, a nonmetal belonging to Group 15 of the periodic table, forms the central atom in the molecule.
-
Five chlorine (Cl) atoms: Chlorine, a highly electronegative halogen, surrounds the central phosphorus atom.
This simple formula, however, hides a fascinating complexity in its molecular structure and behavior.
The Molecular Structure: Beyond the Formula
While the formula PCl₅ suggests a simple structure, the actual arrangement of atoms is more intricate. In the gaseous and liquid phases, phosphorus pentachloride exists as a trigonal bipyramidal molecule. This geometry is dictated by the valence shell electron pair repulsion (VSEPR) theory. The phosphorus atom is surrounded by five chlorine atoms, with three chlorine atoms positioned equatorially and two occupying axial positions. This structure leads to two different types of P-Cl bonds:
-
Axial bonds: These bonds are longer and weaker due to greater repulsion from the equatorial chlorine atoms.
-
Equatorial bonds: These bonds are shorter and stronger due to less repulsion.
This distinction in bond lengths and strengths is crucial in understanding the reactivity of PCl₅.
Solid-State Structure: The Ionic Perspective
The story doesn't end there. In the solid state, phosphorus pentachloride undergoes a fascinating transformation. It exists not as discrete PCl₅ molecules, but as an ionic compound with the formula [PCl₄]+[PCl₆]−. This means it exists as a lattice structure consisting of tetrahedral [PCl₄]+ cations and octahedral [PCl₆]− anions. This solid-state structure highlights the versatility of phosphorus's bonding capabilities. The transition from the molecular structure in the gaseous and liquid phases to the ionic structure in the solid phase illustrates the influence of intermolecular forces and packing efficiency.
Chemical Properties: Reactivity and Applications
The chemical properties of phosphorus pentachloride are directly linked to its molecular and solid-state structures. Its reactivity stems from the polar P-Cl bonds and the presence of readily available electron pairs on the chlorine atoms. Some key properties and reactions include:
Hydrolysis: Reaction with Water
PCl₅ reacts vigorously with water, undergoing hydrolysis to produce phosphoric acid (H₃PO₄) and hydrogen chloride (HCl):
PCl₅ + 4H₂O → H₃PO₄ + 5HCl
This reaction is highly exothermic and generates significant heat.
Reaction with Alcohols: Formation of Alkyl Chlorides
Phosphorus pentachloride reacts with alcohols to produce alkyl chlorides. This reaction is widely used in organic chemistry for the synthesis of various chlorinated compounds:
ROH + PCl₅ → RCl + POCl₃ + HCl
where R represents an alkyl group.
Reaction with Carboxylic Acids: Formation of Acid Chlorides
PCl₅ is a powerful reagent for converting carboxylic acids into acid chlorides. Acid chlorides are versatile intermediates in organic synthesis:
RCOOH + PCl₅ → RCOCl + POCl₃ + HCl
This reaction involves the replacement of the hydroxyl (-OH) group of the carboxylic acid with a chlorine atom.
Reaction with Amines: Formation of Amides
Phosphorus pentachloride can react with primary amines to yield amides. However, the reaction is often complex and may require careful control of reaction conditions:
RNH₂ + PCl₅ → RCONH₂ + ... (The products may vary depending on reaction conditions)
Lewis Acid Behavior: Accepting Electron Pairs
PCl₅ can act as a Lewis acid, meaning it can accept electron pairs from other molecules or ions. This behavior is a consequence of the phosphorus atom's ability to expand its octet. The interaction with Lewis bases leads to the formation of adducts.
Industrial Applications: A Versatile Compound
The versatility of phosphorus pentachloride translates into its diverse applications across various industries. Some key uses include:
-
Chlorinating Agent: Its primary use is as a chlorinating agent in organic and inorganic chemistry, facilitating the introduction of chlorine atoms into molecules. This is crucial in synthesizing various pharmaceutical intermediates, pesticides, and other chemical products.
-
Catalyst: Phosphorus pentachloride acts as a catalyst in certain chemical reactions, speeding up the reaction rate without being consumed itself.
-
Dehydrating Agent: Due to its affinity for water, it can function as a dehydrating agent in specific chemical processes.
Safety Precautions: Handling Phosphorus Pentachloride
Phosphorus pentachloride is a corrosive and reactive chemical compound that requires careful handling. Exposure to the substance can cause severe burns to skin and eyes, and inhalation can lead to respiratory irritation. Therefore, appropriate safety measures are crucial:
-
Personal Protective Equipment (PPE): Always wear appropriate PPE, including safety goggles, gloves, and a lab coat, when handling PCl₅.
-
Ventilation: Work in a well-ventilated area or under a fume hood to minimize inhalation risk.
-
Storage: Store PCl₅ in a tightly sealed container in a cool, dry place away from incompatible materials.
-
Emergency Procedures: Be familiar with emergency procedures in case of accidental exposure.
Conclusion: A Deeper Understanding of PCl₅
This detailed exploration of phosphorus pentachloride has revealed the multifaceted nature of this important chemical compound. From its simple formula, PCl₅, to its intricate molecular and solid-state structures, its diverse reactivity, and widespread industrial applications, PCl₅ exemplifies the complexities and practical significance of chemical compounds. Understanding its properties and handling it safely is essential for anyone working with or studying this versatile substance. The applications detailed above only scratch the surface; ongoing research continues to uncover new and exciting uses for this remarkable chemical. Further investigation into its reactions and catalytic properties will undoubtedly lead to even more advancements in various fields.
Latest Posts
Latest Posts
-
Words That End With A S
May 09, 2025
-
25 Is 50 Of What Number
May 09, 2025
-
What Is 9 Percent In Decimal Form
May 09, 2025
-
5 Letter Word Containing S And I
May 09, 2025
-
Difference Between Experimental Probability And Theoretical Probability
May 09, 2025
Related Post
Thank you for visiting our website which covers about What Is The Formula Of Phosphorus Pentachloride . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.