What Is The Electronic Configuration Of Calcium
Juapaving
Apr 07, 2025 · 6 min read
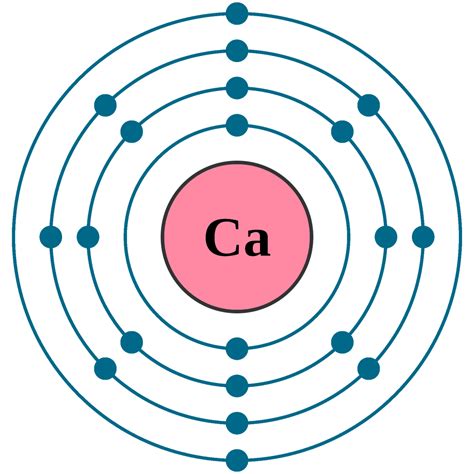
Table of Contents
What is the Electronic Configuration of Calcium? A Deep Dive into Atomic Structure
Calcium, a vital element for life, plays a crucial role in various biological processes. Understanding its electronic configuration is key to comprehending its chemical behavior and biological significance. This article provides a comprehensive exploration of calcium's electronic configuration, delving into the underlying principles of atomic structure and offering practical applications of this knowledge.
Understanding Electronic Configuration
Before diving into calcium's specific configuration, let's establish a foundational understanding of what electronic configuration actually means. The electronic configuration of an atom describes how electrons are distributed among various energy levels (shells) and sublevels (subshells) within the atom. This distribution is governed by several fundamental principles:
The Aufbau Principle
The Aufbau principle, German for "building-up principle," dictates that electrons first fill the lowest energy levels available before occupying higher energy levels. This is analogous to filling a building from the ground floor upwards.
Hund's Rule
Hund's rule states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This minimizes electron-electron repulsion, leading to a more stable configuration. Think of it as ensuring each student gets their own desk before sharing.
Pauli Exclusion Principle
The Pauli exclusion principle asserts that no two electrons within an atom can have the same set of four quantum numbers. This means each orbital can hold a maximum of two electrons, with opposite spins. It's like a rule that each apartment can only house two people.
Determining Calcium's Electronic Configuration
Calcium (Ca) has an atomic number of 20, meaning it possesses 20 protons and, in its neutral state, 20 electrons. To determine its electronic configuration, we systematically fill electron shells and subshells according to the Aufbau principle, Hund's rule, and the Pauli exclusion principle.
The order of filling electron subshells is: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p… and so on. Each subshell has a specific capacity for electrons:
- s subshell: Holds a maximum of 2 electrons
- p subshell: Holds a maximum of 6 electrons
- d subshell: Holds a maximum of 10 electrons
- f subshell: Holds a maximum of 14 electrons
Following these rules, we can systematically fill the orbitals for calcium:
- 1s²: The first shell (n=1) has one subshell (s), which holds 2 electrons.
- 2s²: The second shell (n=2) begins with the s subshell, holding another 2 electrons.
- 2p⁶: The second shell also has a p subshell, which can accommodate 6 electrons.
- 3s²: The third shell (n=3) starts with the s subshell, holding 2 electrons.
- 3p⁶: The third shell continues with the p subshell, holding another 6 electrons.
- 4s²: Finally, the fourth shell (n=4) begins with the s subshell, and the remaining two electrons fill this subshell.
Therefore, the complete electronic configuration of calcium is 1s²2s²2p⁶3s²3p⁶4s². This can also be represented in a shorthand notation using the noble gas configuration: [Ar]4s², where [Ar] represents the electronic configuration of Argon (1s²2s²2p⁶3s²3p⁶).
Significance of Calcium's Electronic Configuration
Calcium's electronic configuration is not just a theoretical exercise; it has profound implications for its chemical and biological properties:
Chemical Reactivity
The outermost shell, also known as the valence shell, determines an element's chemical reactivity. Calcium has two electrons in its valence shell (4s²). These two electrons are relatively loosely held and are readily lost to achieve a stable octet configuration, similar to that of the noble gas Argon. This tendency to lose electrons makes calcium highly reactive, particularly with nonmetals like oxygen and chlorine. This reactivity is crucial in the formation of numerous calcium compounds.
Formation of Ionic Compounds
Calcium's tendency to lose its two valence electrons readily results in the formation of a +2 cation (Ca²⁺). This positively charged ion readily forms ionic compounds with negatively charged anions. For example, calcium readily reacts with oxygen to form calcium oxide (CaO), a stable ionic compound where the calcium cation (Ca²⁺) is electrostatically bonded to the oxide anion (O²⁻). This principle governs many of calcium's interactions within biological systems.
Biological Roles of Calcium
Calcium's reactivity and ability to form stable ionic compounds are central to its crucial roles in biological systems. Some of these roles include:
-
Bone and Teeth Formation: Calcium is the primary mineral component of bones and teeth, providing structural strength and rigidity. It forms strong ionic bonds with phosphate ions to create hydroxyapatite, the primary mineral in bone tissue.
-
Muscle Contraction: Calcium ions (Ca²⁺) are essential for muscle contraction. They trigger the interaction between actin and myosin filaments, leading to muscle shortening.
-
Nerve Impulse Transmission: Calcium ions play a key role in the transmission of nerve impulses across synapses. They mediate the release of neurotransmitters, enabling communication between nerve cells.
-
Blood Clotting: Calcium is a crucial cofactor in the blood clotting cascade. It participates in several enzymatic reactions that lead to the formation of fibrin, the protein responsible for blood clot formation.
-
Enzyme Activation: Many enzymes require calcium ions as cofactors for their activity. Calcium's presence is essential for the proper functioning of these enzymes and many vital metabolic processes.
Beyond the Basics: Deeper Exploration of Calcium's Electronic Structure
While the basic electronic configuration provides a good overview, a more nuanced understanding requires examining aspects like:
Orbital Shapes and Energies
The electronic configuration describes the occupancy of orbitals, but doesn't fully illustrate their shapes and relative energies. The s orbitals are spherical, p orbitals are dumbbell-shaped, and d and f orbitals possess more complex shapes. The relative energies of orbitals can vary depending on the atomic number and the surrounding electronic environment. This variation in energy levels influences the chemical behavior and reactivity of the atom.
Ionization Energies
Ionization energy is the energy required to remove an electron from an atom or ion. Calcium's low first and second ionization energies reflect the relative ease with which it loses its two valence electrons to form the Ca²⁺ ion. The significantly higher third ionization energy illustrates the increased difficulty in removing an electron from the stable, filled electron shells.
Spectroscopic Analysis
Spectroscopic techniques can provide detailed information about the electronic structure of calcium. By analyzing the light emitted or absorbed by calcium atoms, scientists can obtain precise measurements of energy levels and transitions between different electronic states. This provides experimental verification of theoretical predictions about the atom's electronic configuration.
Practical Applications and Conclusion
Understanding the electronic configuration of calcium has significant practical applications in various fields:
-
Material Science: The knowledge of calcium's electronic structure guides the development of new materials with specific properties, such as biocompatible materials for implants and new catalysts.
-
Medicine: Understanding calcium's biological roles helps in the development of new drugs and treatments for calcium-related disorders like osteoporosis and hypocalcemia.
-
Agriculture: Knowledge about calcium's role in plant growth informs agricultural practices aimed at optimizing crop yields and improving soil health.
In conclusion, the electronic configuration of calcium – 1s²2s²2p⁶3s²3p⁶4s² or [Ar]4s² – is not merely a theoretical concept. It provides a fundamental framework for understanding its chemical reactivity, its ability to form ionic compounds, and its crucial biological roles. By delving into the principles that govern electronic configurations and exploring the implications of calcium's specific configuration, we gain a deeper appreciation for the atom's significance in the natural world and its myriad applications in scientific and technological advancements. The seemingly simple arrangement of electrons within the calcium atom holds the key to understanding a vast array of its properties and functionalities.
Latest Posts
Latest Posts
-
Do Gasses Have A Definite Volume
Apr 07, 2025
-
Choose The Components Of A Respiratory Membrane
Apr 07, 2025
-
Why Is The Earth Called The Blue Planet
Apr 07, 2025
-
How Many Electrons Does Oxygen Have In Its Outermost Shell
Apr 07, 2025
-
What Is Not Necessary For Photosynthesis
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electronic Configuration Of Calcium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.