What Is The Electron Configuration Of Ti
Juapaving
Mar 15, 2025 · 7 min read
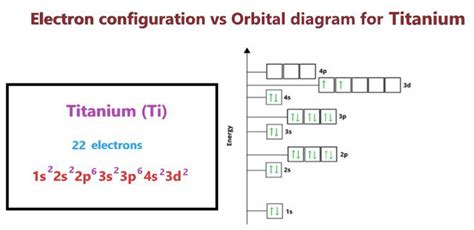
Table of Contents
What is the Electron Configuration of Titanium? A Deep Dive into Atomic Structure
Titanium (Ti), a lustrous transition metal with a silver-grey appearance, holds a fascinating place in the periodic table. Understanding its electron configuration is key to grasping its unique chemical and physical properties, from its high strength-to-weight ratio to its resistance to corrosion. This article will delve deep into the electron configuration of titanium, explaining its derivation, implications, and relevance in various fields.
Understanding Electron Configuration
Before we delve into the specifics of titanium, let's establish a foundational understanding of electron configuration. Electron configuration describes the arrangement of electrons in the various energy levels and sublevels within an atom. It's a crucial concept because the arrangement of electrons dictates an atom's chemical behavior, its reactivity, and its bonding characteristics. These electrons occupy orbitals, regions of space around the nucleus where there's a high probability of finding an electron.
Each electron is assigned to a specific energy level (n), represented by numbers (1, 2, 3, etc.), and a sublevel (s, p, d, f), which further defines the shape and energy of the orbital within that level. The filling of these orbitals follows specific rules, including the Aufbau principle, the Pauli exclusion principle, and Hund's rule.
- Aufbau Principle: Electrons fill orbitals starting from the lowest energy level and progressing to higher energy levels.
- Pauli Exclusion Principle: Each orbital can hold a maximum of two electrons, each with opposite spins.
- Hund's Rule: Within a sublevel, electrons will individually occupy each orbital before pairing up in the same orbital.
Determining the Electron Configuration of Titanium (Ti)
Titanium has an atomic number of 22, meaning it has 22 protons and 22 electrons in a neutral atom. To determine its electron configuration, we follow the Aufbau principle and fill the orbitals according to their energy levels:
1s<sup>2</sup> 2s<sup>2</sup> 2p<sup>6</sup> 3s<sup>2</sup> 3p<sup>6</sup> 4s<sup>2</sup> 3d<sup>2</sup>
Let's break this down:
- 1s<sup>2</sup>: The first energy level (n=1) contains one sublevel, 's', which can hold up to two electrons. Titanium's two lowest-energy electrons occupy this orbital.
- 2s<sup>2</sup>: The second energy level (n=2) also has an 's' sublevel, holding another two electrons.
- 2p<sup>6</sup>: The second energy level also contains a 'p' sublevel, which can hold up to six electrons (three orbitals, each holding two electrons). These six electrons fill the 2p sublevel.
- 3s<sup>2</sup>: The third energy level (n=3) begins with an 's' sublevel, holding two more electrons.
- 3p<sup>6</sup>: The 'p' sublevel in the third energy level holds six electrons.
- 4s<sup>2</sup>: Before filling the 3d sublevel, the 4s sublevel is filled first (due to subtle energy level differences). This orbital holds two more electrons.
- 3d<sup>2</sup>: Finally, the two remaining electrons occupy the 3d sublevel.
Therefore, the complete electron configuration of titanium is 1s<sup>2</sup> 2s<sup>2</sup> 2p<sup>6</sup> 3s<sup>2</sup> 3p<sup>6</sup> 4s<sup>2</sup> 3d<sup>2</sup>. This configuration highlights the presence of two electrons in the 4s orbital and two electrons in the 3d orbital, crucial for understanding titanium's properties.
Noble Gas Configuration: A Simplified Representation
A more concise way to represent the electron configuration of titanium is using the noble gas configuration. Noble gases, with their full valence shells, represent a stable electron configuration. We can use the noble gas preceding titanium, Argon (Ar), which has the electron configuration 1s<sup>2</sup> 2s<sup>2</sup> 2p<sup>6</sup> 3s<sup>2</sup> 3p<sup>6</sup>. Thus, the noble gas configuration of titanium is:
[Ar] 4s<sup>2</sup> 3d<sup>2</sup>
This notation simplifies the representation by showing only the electrons beyond the stable Argon core.
Implications of Titanium's Electron Configuration
Titanium's electron configuration directly influences its properties and behavior:
-
Metallic Character: The presence of electrons in the outer 4s and 3d orbitals contributes to titanium's metallic character. These loosely held electrons are readily available for metallic bonding, resulting in its high electrical and thermal conductivity.
-
Reactivity: While relatively unreactive compared to some other transition metals, titanium's partially filled d orbitals allow for its participation in chemical reactions. It forms stable compounds with various elements, although its strong affinity for oxygen often leads to the formation of a protective oxide layer, contributing to its corrosion resistance.
-
Strength and Lightweight: The strong metallic bonding contributes to titanium's remarkable high strength-to-weight ratio. This makes it a highly sought-after material in aerospace, biomedical, and sporting goods applications.
-
Alloy Formation: The ability of titanium to form alloys with other elements further enhances its properties. Alloying with other metals modifies its strength, ductility, and other characteristics, leading to a wide range of applications.
-
Catalysis: Titanium compounds can act as catalysts in various chemical reactions due to the presence of the d orbitals. They participate in reactions involving oxygen and other elements, particularly in organic chemistry.
Titanium in Various Applications
The unique properties stemming from its electron configuration make titanium a versatile element used in a broad spectrum of applications:
-
Aerospace Industry: Titanium alloys are integral components in aircraft engines, airframes, and spacecraft due to their high strength-to-weight ratio and resistance to high temperatures and corrosion.
-
Biomedical Implants: Its biocompatibility makes titanium ideal for manufacturing prosthetic devices, dental implants, and other medical implants. The body readily accepts titanium, and its resistance to corrosion ensures long-term functionality.
-
Sporting Goods: Titanium's lightweight and high strength make it an excellent material for sporting equipment like golf clubs, bicycle frames, and tennis rackets.
-
Chemical Processing: Its corrosion resistance makes it valuable in chemical processing equipment that handles corrosive substances.
-
Jewelry: Titanium's distinctive silver-grey color and durability make it a popular choice for jewelry items.
Excited States and Ionization
The electron configuration discussed above represents titanium in its ground state, the lowest energy state. However, when titanium absorbs energy, an electron can be promoted to a higher energy level, resulting in an excited state. These excited states are unstable and the electron will quickly return to its ground state, often emitting energy in the form of light.
Ionization occurs when an electron is completely removed from an atom, forming a positively charged ion. Titanium can lose electrons to form ions with various charges (e.g., Ti<sup>2+</sup>, Ti<sup>3+</sup>, Ti<sup>4+</sup>). The ease with which titanium loses electrons is related to the relatively high energy of its 4s and 3d electrons.
The most common ionization states are Ti<sup>2+</sup> and Ti<sup>4+</sup>. The electron configurations of these ions would be:
- Ti<sup>2+</sup>: [Ar] 3d<sup>2</sup> (two 4s electrons are lost)
- Ti<sup>4+</sup>: [Ar] (all 4s and 3d electrons are lost)
Conclusion
The electron configuration of titanium, [Ar] 4s<sup>2</sup> 3d<sup>2</sup>, is the foundation for understanding its remarkable properties and diverse applications. This arrangement of electrons dictates its metallic nature, reactivity, strength, and biocompatibility. By grasping this fundamental aspect of titanium's atomic structure, we can appreciate its significance across various fields, from aerospace engineering to biomedical applications. Further research into titanium's electronic structure continues to unveil new possibilities and expand its use in innovative technologies. The remarkable versatility of this transition metal is intrinsically linked to the unique arrangement of its electrons within its atomic orbitals, making it a truly fascinating element to study.
Latest Posts
Latest Posts
-
Least Common Multiple Of 2 3 And 7
Mar 15, 2025
-
Lcm Of 6 9 And 12
Mar 15, 2025
-
1 Out Of 7 Is What Percentage
Mar 15, 2025
-
The Amount Of Matter In An Object Is Called
Mar 15, 2025
-
Which Of The Following Are Characteristics Of Skeletal Muscle
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration Of Ti . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
