What Is The Electron Configuration Of Rb
Juapaving
Mar 29, 2025 · 5 min read
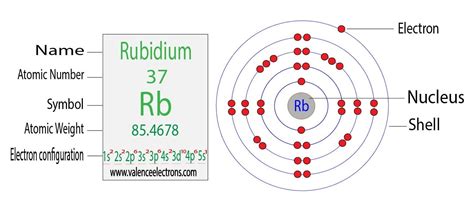
Table of Contents
What is the Electron Configuration of Rb? A Deep Dive into Rubidium's Atomic Structure
Rubidium (Rb), a fascinating alkali metal, holds a unique place in the periodic table. Understanding its electron configuration is key to unlocking its properties and behavior. This article delves deep into the electron configuration of rubidium, exploring its underlying principles, implications, and practical applications. We'll unravel the mysteries behind its atomic structure, providing a comprehensive guide for students and enthusiasts alike.
Understanding Electron Configuration
Before we dive into rubidium's specific configuration, let's establish a foundational understanding of what electron configuration represents. An electron configuration describes the arrangement of electrons in the various energy levels (shells) and sublevels (subshells) within an atom. This arrangement dictates an element's chemical properties, reactivity, and its position within the periodic table. It follows specific rules and principles governed by quantum mechanics.
Key Principles Governing Electron Configuration:
- Aufbau Principle: Electrons fill orbitals starting with the lowest energy levels first. Think of it like filling a building from the ground floor up.
- Pauli Exclusion Principle: Each orbital can hold a maximum of two electrons, each with opposite spins (represented as ↑ and ↓).
- Hund's Rule: Within a subshell, electrons will individually occupy each orbital before pairing up. This minimizes electron-electron repulsion.
Determining the Electron Configuration of Rubidium (Rb)
Rubidium has an atomic number of 37, meaning it possesses 37 protons and, in its neutral state, 37 electrons. To determine its electron configuration, we systematically fill the orbitals according to the Aufbau principle, Pauli exclusion principle, and Hund's rule.
The order of filling orbitals is typically: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p... and so on.
Therefore, the electron configuration of rubidium (Rb) is: 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s¹
Let's break this down:
- 1s²: Two electrons fill the 1s orbital (lowest energy level).
- 2s²: Two electrons fill the 2s orbital.
- 2p⁶: Six electrons fill the three 2p orbitals (px, py, pz).
- 3s²: Two electrons fill the 3s orbital.
- 3p⁶: Six electrons fill the three 3p orbitals.
- 4s²: Two electrons fill the 4s orbital.
- 3d¹⁰: Ten electrons fill the five 3d orbitals.
- 4p⁶: Six electrons fill the three 4p orbitals.
- 5s¹: One electron occupies the 5s orbital. This is the outermost electron and determines rubidium's chemical behavior.
Simplified Notation and Valence Electrons
The electron configuration can be simplified using noble gas notation. Noble gases are group 18 elements with completely filled outer electron shells, making them exceptionally stable. We can represent the core electrons of rubidium using the noble gas preceding it, Krypton (Kr), which has the electron configuration [Kr] = 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶.
Therefore, the simplified electron configuration of rubidium is: [Kr]5s¹
The valence electrons are the electrons in the outermost shell. In rubidium's case, there is only one valence electron (the 5s¹ electron). This single valence electron is responsible for rubidium's high reactivity and its characteristic properties as an alkali metal.
Implications of Rubidium's Electron Configuration
Rubidium's electron configuration directly influences its physical and chemical properties:
-
Reactivity: The single valence electron is easily lost, making rubidium highly reactive. It readily forms a +1 ion (Rb⁺) to achieve a stable noble gas configuration. This explains its vigorous reaction with water and other oxidizing agents.
-
Metallic Character: The presence of a loosely held valence electron contributes to rubidium's metallic character. This electron is delocalized, enabling high electrical and thermal conductivity.
-
Low Ionization Energy: The relatively low energy required to remove the outermost electron (ionization energy) is another consequence of the electron configuration. This further contributes to its high reactivity.
-
Atomic Radius: The size of the rubidium atom is relatively large due to the increasing number of electron shells.
-
Electropositivity: Rubidium is highly electropositive, meaning it readily loses electrons to form positive ions.
Applications Leveraging Rubidium's Properties
The unique properties stemming from its electron configuration make rubidium useful in several applications:
-
Atomic Clocks: Rubidium's precise spectral lines are exploited in atomic clocks for accurate timekeeping.
-
Photoelectric Cells: Its ability to readily emit electrons when exposed to light makes it suitable for photoelectric cells.
-
Catalysis: Rubidium compounds can act as catalysts in certain chemical reactions.
-
Medical Imaging: Specific rubidium isotopes are used in medical imaging techniques.
Beyond the Basics: Exploring Excited States
The electron configuration we've discussed refers to the ground state of rubidium, the lowest energy state. However, when energy is supplied (e.g., through heating or irradiation), electrons can jump to higher energy levels, resulting in an excited state. These excited states are unstable and the electrons quickly return to their ground state, often emitting light in the process. This is the principle behind atomic spectroscopy, a technique used to identify elements based on their unique emission spectra.
Conclusion: Rubidium's Electron Configuration – A Comprehensive Summary
The electron configuration of rubidium, 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s¹ or its simplified version [Kr]5s¹, is fundamental to understanding its behavior and applications. This configuration explains its high reactivity, metallic character, and suitability for specific technological applications. Understanding the principles of electron configuration, such as the Aufbau principle, Pauli exclusion principle, and Hund's rule, provides a solid foundation for comprehending the properties of all elements in the periodic table. The seemingly simple arrangement of electrons within an atom dictates a vast array of chemical and physical characteristics, making the study of electron configuration essential in the realm of chemistry and physics.
Latest Posts
Latest Posts
-
How Can The Strength Of Electromagnet Be Increased
Apr 01, 2025
-
Is 20 A Multiple Of 10
Apr 01, 2025
-
What Are Rows In Periodic Table
Apr 01, 2025
-
Sound Waves Cannot Travel In A Vacuum Because
Apr 01, 2025
-
What Is The Difference Between Place And Value
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration Of Rb . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
