What Is The Electron Configuration Of Germanium
Juapaving
Mar 13, 2025 · 6 min read
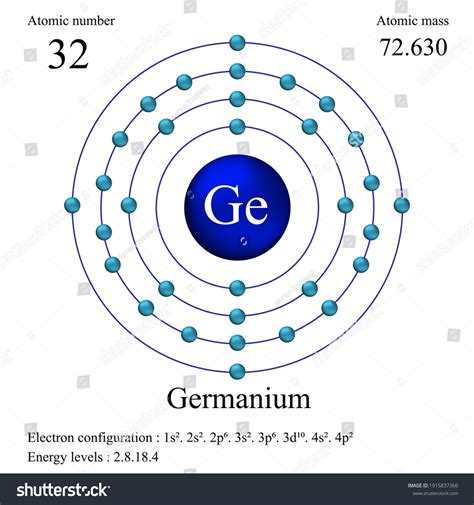
Table of Contents
What is the Electron Configuration of Germanium? A Deep Dive into Atomic Structure
Germanium, a metalloid element with intriguing properties, finds applications in various fields, from semiconductors to fiber optics. Understanding its atomic structure, particularly its electron configuration, is crucial to grasping its behavior and applications. This article provides a comprehensive exploration of germanium's electron configuration, delving into the underlying principles of atomic structure and its implications for germanium's unique characteristics.
Understanding Electron Configuration
Before diving into germanium's specific configuration, let's establish a foundational understanding of electron configuration itself. An element's electron configuration describes how electrons are distributed among the various energy levels and sublevels within an atom. This distribution dictates an element's chemical properties, reactivity, and bonding behavior.
Electrons occupy orbitals, regions of space surrounding the nucleus where there's a high probability of finding an electron. These orbitals are grouped into shells (represented by the principal quantum number, n), which increase in energy as their distance from the nucleus increases. Each shell contains subshells (represented by the azimuthal quantum number, l), denoted by s, p, d, and f, each with a specific number of orbitals and capable of holding a specific number of electrons.
- s subshell: Holds a maximum of 2 electrons.
- p subshell: Holds a maximum of 6 electrons.
- d subshell: Holds a maximum of 10 electrons.
- f subshell: Holds a maximum of 14 electrons.
The Aufbau principle, Hund's rule, and the Pauli exclusion principle govern the filling of these orbitals. The Aufbau principle dictates that electrons fill orbitals from the lowest energy level to the highest. Hund's rule states that electrons fill orbitals individually before pairing up. Finally, the Pauli exclusion principle states that no two electrons in an atom can have the same set of four quantum numbers.
Deriving Germanium's Electron Configuration
Germanium (Ge) has an atomic number of 32, meaning it has 32 protons and 32 electrons in a neutral atom. Following the Aufbau principle, we systematically fill the orbitals:
- 1s²: The first shell (n=1) contains only the s subshell, which holds 2 electrons.
- 2s²: The second shell (n=2) starts with the s subshell, accommodating another 2 electrons.
- 2p⁶: The p subshell in the second shell can hold up to 6 electrons, completely filling it.
- 3s²: The third shell (n=3) begins with the s subshell, holding 2 electrons.
- 3p⁶: The p subshell in the third shell is filled with 6 electrons.
- 4s²: The fourth shell (n=4) starts with the s subshell, holding 2 electrons.
- 3d¹⁰: Note that the 3d subshell, while belonging to the third shell, has a higher energy level than the 4s subshell. Therefore, it's filled next, holding 10 electrons.
- 4p²: Finally, the p subshell in the fourth shell contains the remaining 2 electrons.
Therefore, the complete electron configuration of germanium is: 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p².
This can also be written in a condensed or shorthand notation using the noble gas configuration: [Ar] 3d¹⁰4s²4p², where [Ar] represents the electron configuration of Argon (1s²2s²2p⁶3s²3p⁶). This shorthand notation simplifies the representation while retaining all the essential information.
Implications of Germanium's Electron Configuration
Germanium's electron configuration is directly responsible for its properties and applications. The presence of four valence electrons (the electrons in the outermost shell, 4s²4p²) is particularly significant. These valence electrons participate in chemical bonding, contributing to germanium's ability to form both covalent and metallic bonds.
-
Semiconductor Properties: The four valence electrons allow germanium to form a diamond-like crystal structure, where each germanium atom is covalently bonded to four neighboring atoms. However, the relatively weak bonding and the availability of energy levels close to the valence band allow for the movement of electrons under certain conditions – leading to its semiconductor characteristics. This is crucial for its use in transistors and other semiconductor devices.
-
Chemical Reactivity: Germanium exhibits a moderate level of chemical reactivity. It can form compounds with various elements, though it's less reactive than many other elements in its period. Its ability to form covalent bonds is evident in the existence of germanium halides (GeCl₄, GeBr₄, etc.), oxides (GeO₂, GeO), and other compounds.
-
Applications: Germanium's unique properties, derived from its electron configuration, have led to a variety of applications:
- Semiconductors: Transistors, diodes, and integrated circuits rely on germanium's semiconducting behavior.
- Fiber Optics: Germanium dioxide (GeO₂) is used in the production of optical fibers, enabling efficient transmission of light signals.
- Infrared Optics: Germanium's transparency to infrared radiation makes it suitable for use in infrared detectors and lenses.
- Alloys: Germanium is added to certain alloys to improve their properties.
Beyond the Basics: Excited States and Ionization
While the ground state electron configuration described above represents the most stable state of a germanium atom, it can also exist in excited states. In excited states, one or more electrons absorb energy and jump to higher energy levels. These transitions are temporary, and the electrons will eventually return to their ground state, emitting energy in the process. This process forms the basis of many spectroscopic techniques used to analyze the atomic structure of elements.
Furthermore, understanding germanium's ionization energies is essential. Ionization energy is the energy required to remove an electron from an atom. Germanium has several ionization energies, corresponding to the removal of successive electrons. The first ionization energy is relatively low compared to elements with similar electron configuration, highlighting the ease with which germanium loses an electron in certain chemical processes. Subsequent ionization energies increase substantially, reflecting the increasing difficulty in removing electrons from the increasingly positively charged ion.
Comparison with Other Elements: Periodic Trends
Analyzing germanium's electron configuration in the context of its periodic table neighbors reveals important trends. Germanium belongs to group 14 (also known as the carbon group), sharing similarities with silicon, tin, and lead. All elements in this group have four valence electrons, explaining the similarities in their chemical bonding and semiconductor properties. However, differences in atomic size, shielding effects, and other factors lead to variations in their specific properties.
For instance, silicon, located above germanium in the periodic table, has similar semiconductor properties but a higher band gap, affecting its electronic behavior. Tin and lead, located below germanium, show more metallic characteristics as their atomic size increases, weakening the covalent bonding and enhancing metallic bonding tendencies.
Conclusion: The Significance of Electron Configuration
The electron configuration of germanium, [Ar] 3d¹⁰4s²4p², is the cornerstone to understanding its physical and chemical characteristics. The four valence electrons, the presence of a filled d subshell, and its position in the periodic table collectively contribute to its unique properties, leading to its widespread applications in various technological fields. By comprehending the underlying principles of atomic structure and the implications of electron configuration, we gain invaluable insights into the behavior of this crucial metalloid element and its importance in modern technology. Further research into germanium's electronic structure continues to reveal new aspects and potential applications, driving advancements in diverse scientific and technological domains.
Latest Posts
Latest Posts
-
Square Root Of 2 As A Fraction
Mar 13, 2025
-
Least Common Multiple Of 8 And 32
Mar 13, 2025
-
Is Mercury A Pure Substance Or Mixture
Mar 13, 2025
-
Photosystem I And Photosystem Ii Are Part Of
Mar 13, 2025
-
Difference Between Facilitated Diffusion And Active Transport
Mar 13, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration Of Germanium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
