What Is The Electron Configuration For Bromine
Juapaving
Apr 06, 2025 · 4 min read
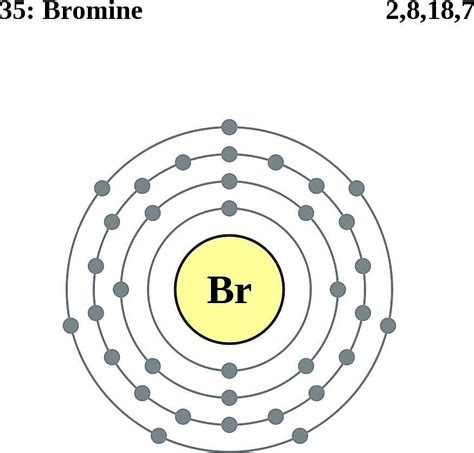
Table of Contents
What is the Electron Configuration for Bromine? A Deep Dive into Atomic Structure
Bromine, a fascinating element with a rich history and diverse applications, holds a unique place in the periodic table. Understanding its electron configuration is key to unlocking its chemical behavior and properties. This comprehensive guide will delve into the intricacies of bromine's electron configuration, exploring its underlying principles, exceptions, and implications.
Understanding Electron Configuration
Before we dive into bromine's specific electron configuration, let's establish a foundational understanding of the concept. Electron configuration describes the arrangement of electrons in an atom's energy levels and sublevels. It's dictated by the principles of quantum mechanics, which govern the behavior of electrons within an atom. This arrangement significantly influences an element's chemical reactivity and bonding properties.
Key Principles Governing Electron Configuration
- Aufbau Principle: This principle dictates that electrons fill the lowest energy levels first, progressing to higher energy levels as they are filled.
- Pauli Exclusion Principle: This principle states that no two electrons in an atom can have the same set of four quantum numbers (n, l, ml, and ms). In simpler terms, each orbital can hold a maximum of two electrons with opposite spins.
- Hund's Rule: This rule states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This minimizes electron-electron repulsion.
These principles are crucial for correctly determining the electron configuration of any element, including bromine.
Determining Bromine's Electron Configuration
Bromine (Br) has an atomic number of 35, meaning it possesses 35 protons and 35 electrons in a neutral atom. Using the Aufbau principle, we systematically fill the electron orbitals:
1s², 2s², 2p⁶, 3s², 3p⁶, 4s², 3d¹⁰, 4p⁵
Let's break down this configuration step-by-step:
- 1s²: The first energy level (n=1) contains one subshell, the s subshell, which can hold a maximum of two electrons.
- 2s²: The second energy level (n=2) also contains an s subshell, holding another two electrons.
- 2p⁶: The second energy level also includes the p subshell, which can hold up to six electrons.
- 3s², 3p⁶: The third energy level (n=3) follows the same pattern with filled s and p subshells.
- 4s²: The fourth energy level (n=4) begins with the filling of the s subshell.
- 3d¹⁰: Note that the 3d subshell, which can hold ten electrons, fills after the 4s subshell. This is because the 4s subshell has a slightly lower energy level than the 3d subshell.
- 4p⁵: Finally, the 4p subshell contains five electrons, incompletely filling this subshell. This incompletely filled p subshell accounts for bromine's high reactivity.
Therefore, the complete electron configuration for bromine is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁵.
Orbital Diagrams and Electron Configuration
Visualizing the electron configuration using orbital diagrams can further enhance our understanding. Each orbital is represented by a box, and electrons are represented by arrows, with opposite spins indicated by up and down arrows. Following Hund's rule, electrons fill orbitals individually before pairing up.
For bromine, the orbital diagram would appear as follows:
(1s) (2s) (2px)(2py)(2pz) (3s) (3px)(3py)(3pz) (4s) (3d)(3d)(3d)(3d)(3d) (4px)(4py)(4pz)
↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓↑↓↑↓↑↓↑↓ ↑↓ ↑ ↑ ↑
This diagram visually confirms the electron configuration we derived earlier.
Bromine's Chemical Behavior and its Electron Configuration
Bromine's electron configuration directly influences its chemical properties and behavior. The presence of five electrons in the 4p subshell makes bromine highly reactive. It readily gains one electron to achieve a stable octet configuration, similar to the noble gas krypton. This explains its strong tendency to form -1 anions (bromide ions, Br⁻) in ionic compounds. It also explains its ability to form covalent bonds by sharing electrons with other atoms.
Exceptions to the Aufbau Principle and Bromine
While the Aufbau principle provides a general guideline, there are exceptions. These exceptions primarily occur in transition metals and some heavier elements due to complex interactions between electrons and orbitals. However, bromine's electron configuration follows the Aufbau principle without deviation.
Conclusion: A Comprehensive Understanding
This detailed exploration of bromine's electron configuration provides a comprehensive understanding of its atomic structure and its influence on its chemical behavior. By applying the fundamental principles of quantum mechanics and using systematic filling of orbitals, we've determined and visualized the electron arrangement in bromine. This knowledge is essential for predicting its reactivity, bonding patterns, and properties within various chemical contexts. Furthermore, understanding electron configurations forms a foundation for studying more complex chemical phenomena and processes. The application of this fundamental knowledge extends far beyond a simple explanation – it unveils the essence of bromine's chemical identity and its interactions within the larger world of chemistry. This detailed breakdown serves as a valuable resource for students and enthusiasts of chemistry seeking a deeper understanding of this fascinating element.
Latest Posts
Latest Posts
-
5 Letter Word Starts With Pro
Apr 07, 2025
-
Is The Square Root Of 24 A Rational Number
Apr 07, 2025
-
2550 50 100 20 100 100 600
Apr 07, 2025
-
Which One Of The Following Statements Is Not True
Apr 07, 2025
-
120 Sq Meters In Sq Feet
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration For Bromine . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.