What Is The Correct Formula For Calcium Oxide
Juapaving
Mar 13, 2025 · 5 min read
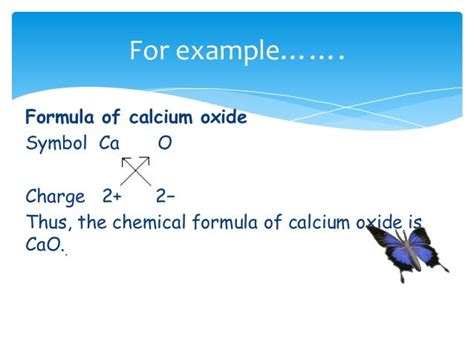
Table of Contents
What is the Correct Formula for Calcium Oxide?
Calcium oxide, commonly known as quicklime or burnt lime, is a widely used chemical compound with a simple yet crucial formula. Understanding its composition is fundamental to comprehending its various applications in diverse industries. This comprehensive guide delves deep into the chemical formula of calcium oxide, exploring its properties, production methods, and significant uses. We'll also address some common misconceptions surrounding its formula.
Understanding the Chemical Formula: CaO
The correct chemical formula for calcium oxide is CaO. This concise notation encapsulates the fundamental building blocks of this compound:
-
Ca: Represents the element calcium, an alkaline earth metal with atomic number 20. Calcium atoms readily lose two electrons to achieve a stable electron configuration.
-
O: Represents the element oxygen, a highly reactive nonmetal with atomic number 8. Oxygen atoms readily gain two electrons to achieve a stable electron configuration.
The combination of one calcium atom (Ca) and one oxygen atom (O) forms an ionic bond. Calcium donates its two valence electrons to oxygen, resulting in the formation of a stable ionic lattice structure. This ionic bond is responsible for the high melting and boiling points of calcium oxide.
Ionic Bonding in Calcium Oxide
The ionic bond in CaO is characterized by a strong electrostatic attraction between the positively charged calcium ion (Ca²⁺) and the negatively charged oxide ion (O²⁻). This strong attraction contributes to the crystalline structure of calcium oxide, a characteristic feature observable in its physical properties. The strong ionic bonds result in a high lattice energy, making it a stable and relatively unreactive compound under normal conditions. However, its reactivity significantly increases in the presence of water or acids.
Production of Calcium Oxide: The Calcination Process
Calcium oxide is primarily produced through the thermal decomposition of calcium carbonate (CaCO₃), commonly found in limestone and chalk. This process is known as calcination. The calcination process involves heating calcium carbonate to a high temperature (typically around 825-900°C), which breaks down the carbonate into calcium oxide and carbon dioxide. The chemical equation for this reaction is:
CaCO₃(s) → CaO(s) + CO₂(g)
This reaction is reversible; however, at high temperatures, the equilibrium shifts towards the right, favoring the formation of calcium oxide and the release of carbon dioxide gas. The efficient removal of CO₂ from the reaction environment further drives the reaction to completion. Industrial kilns are designed to facilitate the efficient removal of CO₂ and maintain the high temperatures required for the calcination process.
Properties of Calcium Oxide
Calcium oxide possesses several key properties that contribute to its widespread use:
-
High Melting Point: CaO has a very high melting point (2572°C), indicating strong ionic bonding within its crystalline structure.
-
Reactivity with Water: Calcium oxide is highly reactive with water, a process known as slaking. This reaction is exothermic, meaning it releases heat:
CaO(s) + H₂O(l) → Ca(OH)₂(s)
The product of this reaction is calcium hydroxide, also known as slaked lime.
-
Alkaline Nature: Calcium oxide is a strong base, readily reacting with acids to form salts and water.
-
High Hardness: Calcium oxide exhibits considerable hardness, a characteristic utilized in certain industrial applications.
-
Hygroscopic: Calcium oxide readily absorbs moisture from the air.
Applications of Calcium Oxide
The versatility of calcium oxide makes it a crucial ingredient in numerous industries:
1. Construction and Building Materials:
-
Cement Production: Calcium oxide is a fundamental component in the production of Portland cement, a key ingredient in concrete. It reacts with other components during the cement hydration process, contributing to the strength and durability of concrete structures.
-
Mortar and Plaster: Slaked lime (calcium hydroxide) derived from calcium oxide is used in the preparation of mortar and plaster, acting as a binder in masonry work.
-
Soil Stabilization: Calcium oxide is employed in soil stabilization to improve its bearing capacity and reduce its permeability.
2. Metallurgical Applications:
-
Flux in Metal Refining: Calcium oxide acts as a flux in metallurgical processes, assisting in removing impurities from molten metals.
-
Steel Production: It plays a role in steel production by reacting with impurities to form slag.
3. Chemical Industry:
-
Production of Calcium Hydroxide: As previously mentioned, the reaction of calcium oxide with water is used to produce calcium hydroxide, a significant chemical in various industrial processes.
-
Neutralization of Acids: Calcium oxide's strong basicity allows it to be used for neutralizing acidic substances.
4. Environmental Applications:
-
Water Treatment: Calcium oxide can be employed in water treatment to adjust the pH and remove impurities.
-
Sulfur Dioxide Removal: Calcium oxide can be used to remove sulfur dioxide (SO₂) from industrial emissions, contributing to air pollution control.
5. Other Applications:
-
Paper Manufacturing: It is used in the paper industry as a pH regulator.
-
Food Industry: Calcium oxide finds limited use as a food additive (E529) in some countries, primarily as an acidity regulator.
-
Agriculture: It is used to improve soil conditions, particularly in acidic soils.
Common Misconceptions about the Formula
It's essential to dispel some common misconceptions about the formula of calcium oxide:
-
Ca₂O: This is incorrect. The correct formula reflects the balanced ionic charges (Ca²⁺ and O²⁻) requiring a 1:1 ratio.
-
CaO₂: This formula represents calcium peroxide, a different compound with different properties and a distinct chemical structure. It contains a peroxide ion (O₂²⁻) instead of an oxide ion (O²⁻).
Conclusion
The correct chemical formula for calcium oxide is unequivocally CaO. Understanding its simple yet profound formula is crucial for comprehending its wide-ranging applications in diverse industries. From building materials to metallurgical processes and environmental applications, calcium oxide plays a pivotal role in modern society. Its properties, including its high reactivity with water and its strong basicity, contribute to its significant utility in various industrial processes and applications. This comprehensive overview underscores the importance of accurate chemical formulas in scientific understanding and practical application. Remember to always double-check your sources and understand the chemical principles behind each formula. This will help you avoid confusion and apply your knowledge effectively.
Latest Posts
Latest Posts
-
Why Was A Stain Added To The Cheek Cells
May 09, 2025
-
How Much Time Is 180 Minutes
May 09, 2025
-
What Is A Group Of Tissues That Work Together Called
May 09, 2025
-
What Is The Difference Between A Square And Rhombus
May 09, 2025
-
1 424 Rounded To The Nearest Hundredth
May 09, 2025
Related Post
Thank you for visiting our website which covers about What Is The Correct Formula For Calcium Oxide . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.